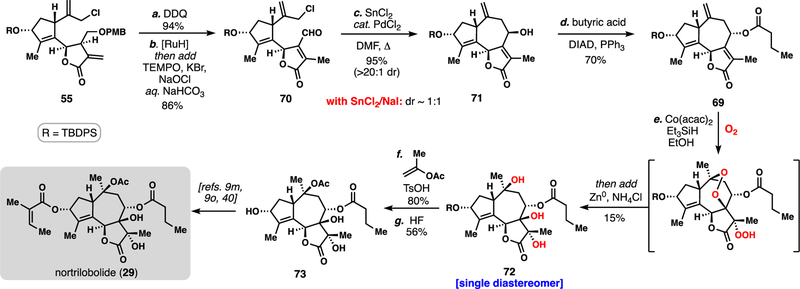
Scheme 4.
12-Step formal synthesis of nortrilobolide (29).a
aReagents and conditions: (a) 55 (1.0 equiv), DDQ (3.0 equiv), pH = 7.5 buffer, DCM, 23 °C, 1 h, 94%; (b) RuHCl(CO)(PPh3)3 (0.1 equiv), DCE, 60 ºC, 16 h, then add TEMPO (0.1 equiv), KBr (1.0 equiv), aq. NaOCl, pH = 8.6 buffer, 23°C, 1 h, 86%; (c) SnCl2 (5.0 equiv), PdCl2(PhCN)2 (0.15 equiv), DMF, 60 ºC, 8 h, 95%; (d) butyric acid (3.0 equiv) DIAD (3.0 equiv), 0 ºC to 23 °C, 8 h, 70%. (e) Co(acac)2 (0.2 equiv), Et3SiH (5.0 equiv), O2 (1 atm), EtOH 24 h, 23 °C, then add Zn (3.0 equiv), aq. NH4Cl (5 equiv), 15%; (f) pTSA (1.2 equiv), isopropenyl acetate, 23 °C, 80%; (g) HF/MeCN (1:5 = v/v), 23 °C, 56%; DDQ = 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone [RuH] = RuHCl(CO)(PPh3)3, acac = acetylacetone.