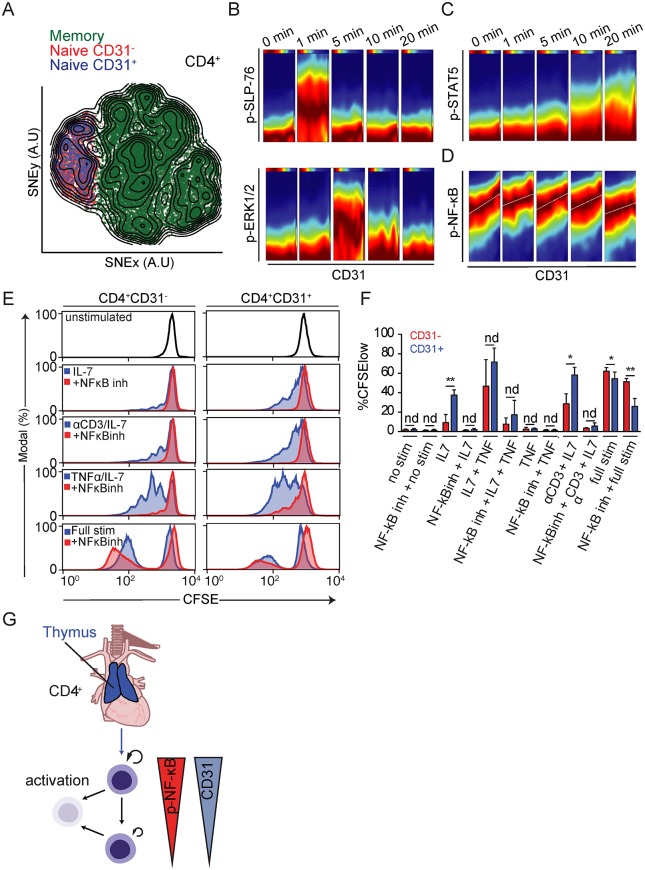
Fig 6. Evidence for a link between replicative history and homeostatic proliferation potential controlled by NF-κB.
(A) Memory/effector (green) and naive CD4+ T cells divided into CD31− (red) and CD31+ (blue) cells were merged and their high-dimensional single-cell phenotypes analyzed by mass cytometry visualized using a tSNE dimensionality reduction. (B) DREVI plots depicting correlation between CD31 and p-SLP-76, p-ERK1/2, (C) p-STAT5, and (D) p-NF-κB throughout a 20-minute homeostatic stimulation with αCD3 and IL-7. Data are representative of four separate experiments on eight donors. (E) Proliferation of sort-purified CD4+CD31− (left) and CD4+CD31+ (right) naive T cells in response to different stimulation conditions (blue histograms) as well as in the presence of the NF-κB inhibitor 6-amino-4–4-phenoxyphenylethylamino-quinazoline (red histograms). Data represent four experiments on eight donors. (F) Summary data from four independent donors showing percent CFSElow (proliferated) cells within the CD31+ and CD31− fraction of naive CD4+ T cells relative to different stimulatory conditions. Cells were treated with vehicle (DMSO) or NF-κB inhibitor (“NFκBinh” 6-amino-4–4-phenoxyphenylethylamino-quinazoline) for each stimulation condition (S3 Data). Full Stim: αCD3/αCD28/IL-2. (G) Schematic summary of the dynamics of CD4+ naive T-cell homeostasis in adult humans. A.U., arbitrary units; CFSE, carboxyfluorescein succinimidyl ester; DREVI, conditional-density rescaled visualization; IL, interleukin; nd, no difference; NF-κB, nuclear factor κB; p-ERK1/2, phosphorylation of extracellular signal–regulated kinase 1/2; p-NF-κB, phosphorylation of NF-κB; p-SLP-76, phosphorylation of SH2 domain-containing leukocyte protein of 76 kilodaltons; p-STAT5, phosphorylation of signal transducer and activator of transcription 5; stim, stimulation; TNF, tumor necrosis factor; tSNE, t-stochastic neighborhood embedding.