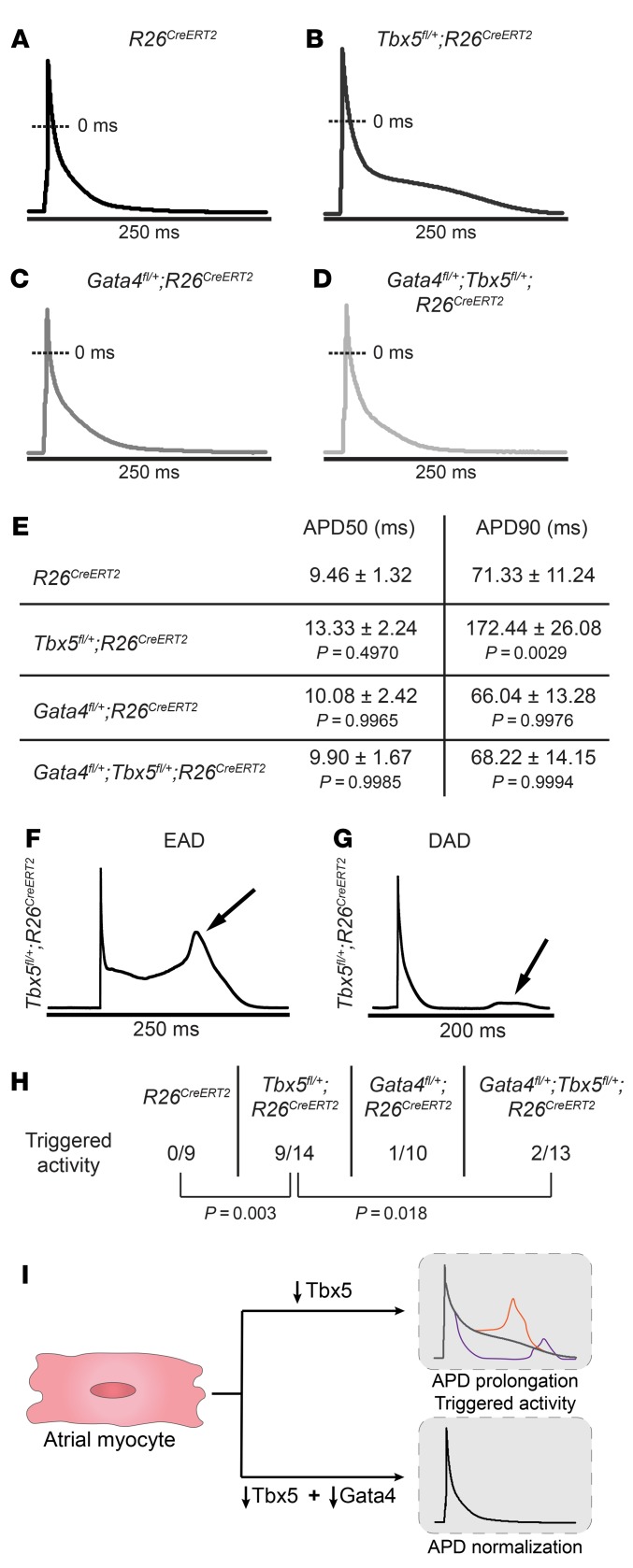
Figure 2. Abnormal atrial cardiomyocyte electrical activity caused by Tbx5 haploinsufficiency is rescued by Gata4 haploinsufficiency.
(A–D) Representative AP recordings from atrial myocytes isolated from R26CreERT2, Tbx5fl/+;R26CreERT2, Gata4fl/+;R26CreERT2, and Gata4fl/+;Tbx5fl/+;R26CreERT2 mice 2 weeks after receiving TM. Prolongation of phase 2 and 3 of the AP was observed exclusively in Tbx5fl/+;R26CreERT2 mice (B) but not in Gata4fl/+;Tbx5fl/+;R26CreERT2 littermates (D), suggesting rescue of AP abnormalities. (E) Corresponding properties of AP from mice in A–D. Tbx5 heterozygotes showed significant prolongation of APD90 in comparison with R26CreERT2 controls. These defects were completely rescued in Gata4/Tbx5 compound heterozygote mice (APD90, P = 0.006). APD50, APD at 50% repolarization; APD90, APD at 90% repolarization. Data represent mean ± SEM (n = 3–5 animals per genotype; n = 9 cardiomyocytes R26CreERT2, n = 14 Tbx5fl/+;R26CreERT2, n = 10 Gata4fl/+;R26CreERT2, and n = 13 Gata4fl/+;Tbx5fl/+;R26CreERT2). P values of APD50 and APD90 were determined by 1-way ANOVA followed by Tukey post-hoc test. (F and G) Representative tracings of EADs and DADs in Tbx5fl/+;R26CreERT2 atrial myocytes. (H) Representative abnormal depolarization events (EADs and DADs) observed in atrial myocytes. Reduced Gata4 gene dosage rescued abnormal triggers observed in Tbx5- haploinsufficient atrial myocytes. Total number of ectopic depolarization events were recorded in R26CreERT2 (n = 9), Tbx5fl/+;R26CreERT2 (n = 14), Gata4fl/+;R26CreERT2 (n = 10), and Gata4fl/+;Tbx5fl/+;R26CreERT2 (n = 13) atrial myocytes from n > 4 for each genotype. P values of triggers were determined by Fisher’s exact test. (I) The cellular conduction deficits caused by Tbx5 haploinsufficiency, including AP prolongation and triggered activity and normalization by reduced Gata4 dose.