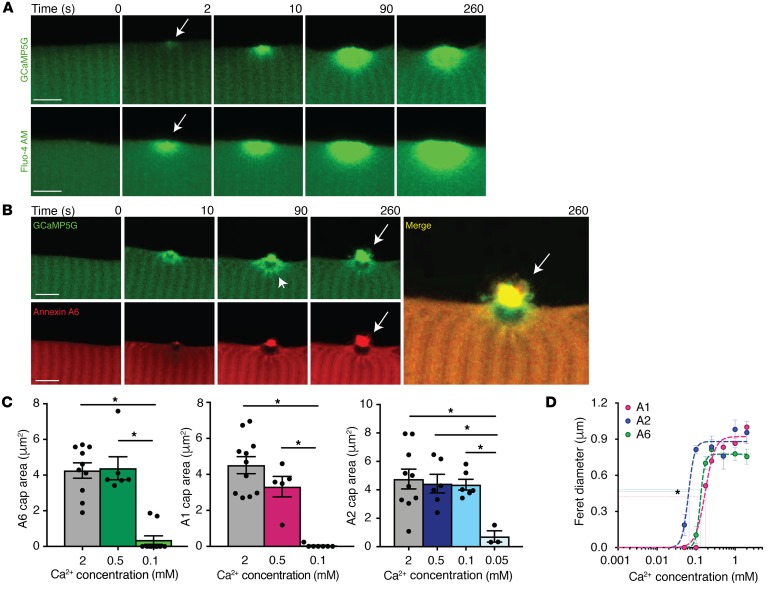
Figure 1. Ca2+-dependent annexin repair cap recruitment at the site of injury.
Myofibers were generated to express the Ca2+ indicator GCaMP5G (green), and time-lapse single-slice images were assessed at time points after membrane disruption. (A) GCaMP5G fluorescence was present at the site of injury, at 2 seconds (arrow), indicating the presence of Ca2+ immediately after damage at the site of injury (top panel). These data were validated with a non–protein-based Ca2+ indicator, Fluo-4 AM (green, bottom panel). (B) Time-lapse images of myofibers coelectroporated with GCaMP5G and annexin A6-tdTomato (A6, red). GCaMP5G fluorescence was present at the site of injury localized around the annexin A6–free zone (arrowhead) and at the annexin A6 cap (arrow). GCaMP5G colocalized (merge, yellow, arrow) with the annexin A6 repair cap. Scale bars: 5 μm. (C) Myofibers expressing fluorescently tagged annexins A1, A2, or A6 were injured at multiple Ca2+ concentrations. Annexin A1 and A6 repair cap size was reduced at 0.1 mM Ca2+ compared with 2 mM and 0.5 mM. Annexin A2 repair cap area was significantly reduced at 0.05 mM Ca2+ compared with 2 mM, 0.5 mM, and 0.1 mM Ca2+. (D) Cap kinetics were plotted as cap Feret diameter over a range of Ca2+ concentrations. Annexin A2 had a statistically significant leftward shift in Km(1/2), followed by annexin A6 and then A1. Data are expressed as mean ± SEM. Differences were tested by 1-way ANOVA with Tukey’s multiple-comparisons test (C). *P < 0.05 (n = 5 myofibers per condition).