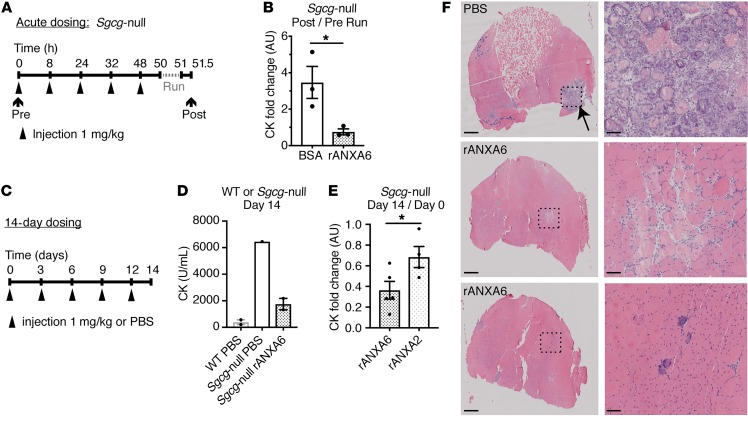
Figure 9. Recombinant annexin A6 protected against muscle damage in a mouse model of muscular dystrophy in vivo.
(A and B) Sgcg-null mice, a model of limb girdle muscular dystrophy 2C, were injected intravenously with recombinant human annexin A6 (rANXA6) or BSA control solution 5 times over 48 hours. Prior to injections, serum creatine kinase (CK) was measured. Two hours after the fifth injection, mice were subjected to 60 minutes of downhill running. Thirty minutes after exercise, serum CK was measured. The fold change in CK after/before running was significantly reduced with rANXA6 administration compared with BSA-injected controls, consistent with a reduction in muscle injury from acute running. (C and D) Sgcg-null mice were injected intravenously every 3 days over 14 days with rANXA6 or control. On day 14, serum CK was evaluated. Serum CK in Sgcg-null mice treated with rANXA6 was lower than PBS control. (E) Sgcg-null mice were injected intravenously every 3 days for 14 days with rANXA6 or recombinant annexin A2. The serum CK fold change after/before treatment (day 14/day 0) was significantly reduced in Sgcg-null mice treated with recombinant annexin A6 compared with annexin A2. (F) Histological analysis of gastrocnemius/soleus muscles from Sgcg-null mice shown in part D injected with PBS or recombinant annexin A6. Low magnification is on the left and high magnification of boxed areas is on the right. Scale bars: 500 μm (left) and 50 μm (right). Data are expressed as mean ± SEM. Differences were assessed by 2-tailed t test (B and E). *P < 0.05 (n = 3 mice per condition, except part D, in which n = 2 WT controls, 1 Sgcg-null control, and 2 Sgcg-null treated mice).