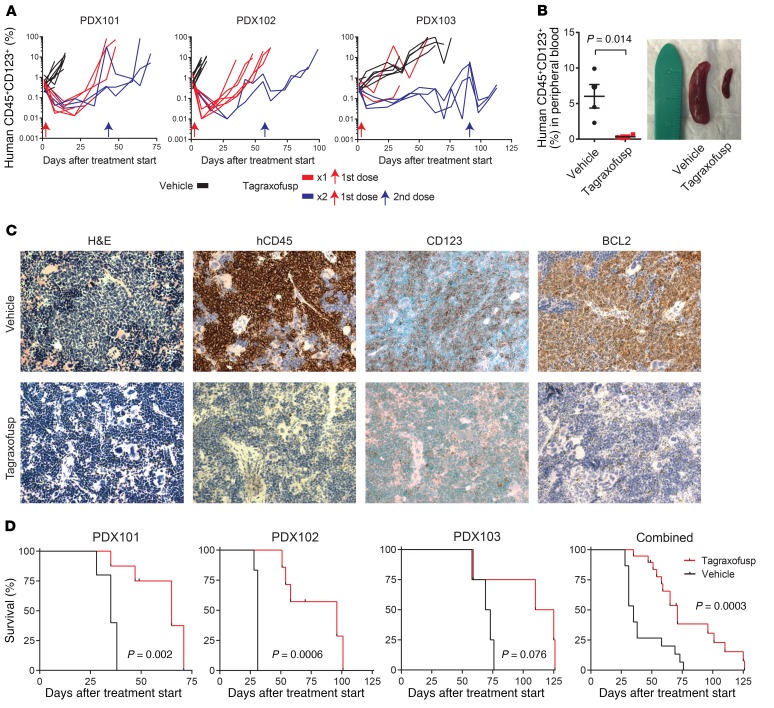
Figure 5. Tagraxofusp is active in BPDCN patient–derived xenografts (PDXs) in vivo.
(A) Human CD45+CD123+ cells as a percentage of the peripheral blood mononuclear cells in NSG mice engrafted with 1 of 3 BPDCN PDXs, treated at day 0 (red arrow) with 5 days of tagraxofusp (red line) or vehicle (black line). A subset of animals in each group was re-treated with another cycle at the time when greater than 50% of animals showed progression (>5% in peripheral blood; blue lines represent animals that received 2 treatments and blue arrows are the time of the second treatment). (B) Peripheral blood disease burden measured by CD45+CD123+ flow cytometry and representative spleen size reduction in animals treated with tagraxofusp as compared to vehicle harvested 7 days after treatment (n = 4 each). (C) Sections from mouse spleens harvested on day 7 after treatment with tagraxofusp or vehicle and stained with hematoxylin & eosin (H&E) or the indicated antibodies by immunohistochemistry. Original magnification, ×40. (D) Kaplan-Meier overall survival curves for recipients of each PDX (left) and for all PDXs combined (right) that received tagraxofusp (red, n = 19) or vehicle (black, n = 15). Curves compared by log-rank test.