Figure. Distribution of the H2FPEF–score in the global TOPCAT trial, and its association with concentrations of natriuretic peptides and the primary composite outcome.
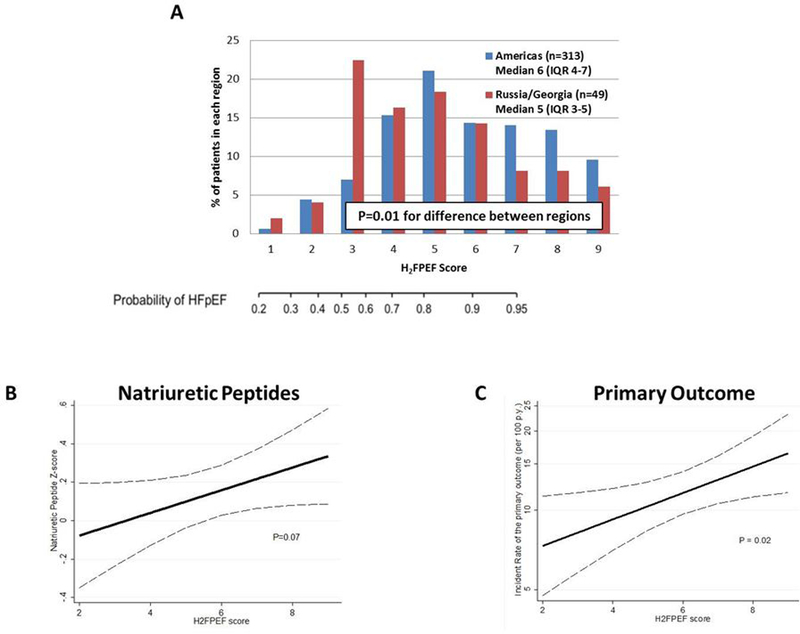
Overall, 362 patients had available data to calculate the H2FPEF-score. Distribution of scores in patients enrolled in the Americas and Russia/Georgia are presented in Panel A. The association between the H2FPEF-score and log-transformed, standardized natriuretic peptide concentrations as a continuous variable is presented in Panel B, and association between the H2FPEF-score and incidence of the primary composite endpoint in Panel C. Natriuretic peptide levels (either B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide) were log-transformed and standardized (expressed per 1 standard deviation; Z-score). The primary outcome for the TOPCAT trial and for this analysis was time to composite hospitalization for heart failure, cardiovascular death, or aborted cardiac arrest. The dotted lines reflect the 95% confidence interval.
