Figure 6.
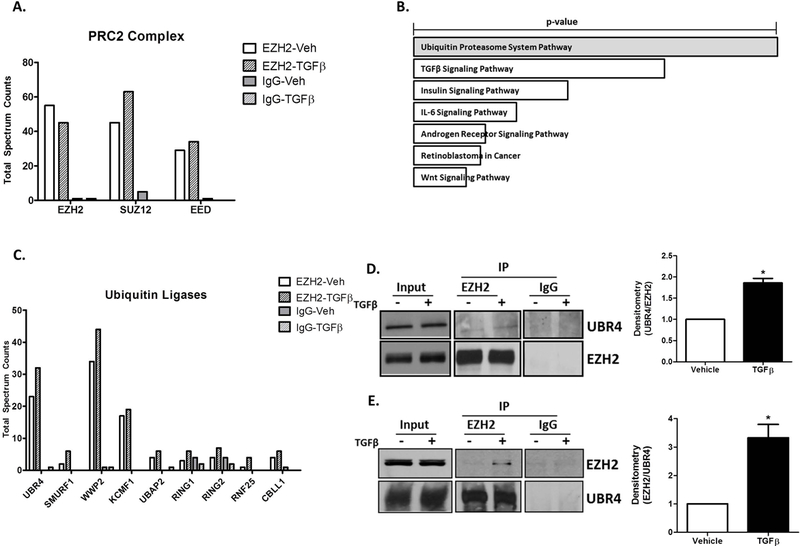
EZH2 is Ubiquitinated by the E3 Ubiquitin Ligase, UBR4.
A. Total Spectrum Counts of PRC2 complex components; EZH2, EED, and SUZ12 identified by mass spectrometry in EZH2 and IgG immunoprecipitates from vehicle and TGFβ treated H69 cells.
B. EnrichR analysis on proteins co-immunoprecipitated with EZH2 identified the Ubiquitin Proteasome System and TGFβ Signaling as top pathways (p-vaue represents Fisher exact test).
C. Total Spectrum Counts of the Ubiquitin ligases co-immunoprecipitated with EZH2 and IgG (control) from H69 cells treated with vehicle or TGFβ. UBR4: Ubiquitin Protein Ligase E3 Component N-Recognin 4, SMURF1: SMAD Specific E3 Ubiquitin Protein Ligase 1, WWP2: WW Domain Containing E3 Ubiquitin Protein Ligase 2, KCMF1: Potassium Channel Modulatory Factor 1, UBAP2: Ubiquitin Associated Protein 2, RING1: Ring Finger Protein 1, RING2: Ring Finger Protein 2, RNF25: Ring Finger Protein 25, CBLL1: Cbl Proto-Oncogene Like 1.
D. Western blotting on EZH2 and IgG immunoprecipitates from H69 cells treated with vehicle or TGFβ confirms the presence of UBR4 (left). Densitometry on the right shows a1.6-fold increase in UBR4 co-immunoprecipitated with EZH2. *p<0.01. All error bars are SEM, n=4.
E. Western blotting on UBR4 and IgG immunoprecipitates from H69 cells treated with vehicle or TGFβ confirms EZH2 in complex with UBR4 (left). Ddensitometric quantification demonstrates a 3-fold increase in EZH2 co-immunoprecipitated with UBR4 after TGF β. *p<0.01. All error bars are SEM, n=4.
