Abstract
Background:
Although skeletal related events (SREs) are linked with reduced quality of life and worse outcomes, factors that predict SREs are minimally understood. We aimed to identify predictors of SREs and all-cause mortality among men with metastatic, castration-resistant prostate cancer (mCRPC).
Methods:
We collected data on 837 men with bone mCRPC at 8 VA centers within the Shared Equal Access Regional Cancer Hospital (SEARCH) database 2000-2017. Patients were followed to assess development of SREs (pathological fracture, radiation to bone, spinal cord compression, surgery to bone). Cox models were used to evaluate predictors of SREs and mortality.
Results:
Of 837 men with bone mCRPC, 287 developed a SRE, and 740 died (median follow-up: 26 months). Bone pain was the strongest predictor of SREs (HR=2.96, 95% CI=2.25-3.89). Shorter time from CRPC to metastasis (HR=0.92, 95%CI=0.85-0.99), shorter progression to CRPC (HR=0.94, 95%CI=0.91-0.98), and visceral metastasis at the time of bone metastasis diagnosis (HR=1.91, 95%CI=1.18-3.09) were associated with increased SREs. Ten or more bone metastases (HR=2.17, 95% CI, 1.72-2.74), receiving radical prostatectomy (HR=0.73, 95%CI=0.61-0.89), shorter progression to CRPC (HR=0.97, 95%CI=0.94-0.99), older age (HR=1.03, 95%CI=1.02-1.04), higher PSA at metastasis (HR=1.21, 95%CI=1.14-1.28), bone pain (HR=1.44, 95%CI=1.23-1.70), and visceral metastasis (HR=1.72, 95%CI=1.23-2.39), were associated with increased mortality risk.
Conclusions:
Among men with bone mCRPC, bone pain was the strongest predictor for SREs and number of bone metastases was a strong predictor for mortality. If validated, these factors may potentially be used for risk stratification and for SRE prevention strategies.
Keywords: Prostate Cancer, Metastasis, Bone, Skeletal Events, Predictors, SEARCH
Precis
The strongest predictor for SRE development was bone pain (HR 2.96, 95%CI 2.25-3.89). Moreover, at least ten bone metastases at time of bone metastasis diagnosis (HR=2.17, 95% CI, 1.72-2.74) resulted in significantly worse overall survival.
Introduction
For men in the United States, prostate cancer is the most common malignancy and the second most common cancer-related cause of death1. In 2018, there will be approximately 164,690 diagnoses and 29,430 deaths from prostate cancer in the United States2. Prostate cancer deaths are typically characterized by progressive metastatic, castration resistant prostate cancer (mCRPC) 3, 4. More than 90% of men with mCRPC develop bone metastases and as such are subsequently at higher risk of skeletal-related events (SREs) which may include pathological fractures and spinal cord compression5-7. The disease burden of prostate cancer and SREs are associated with significant declines in quality of life, poorer survival outcomes, and increased health care costs8-11. Consequently, it is important to identify at-risk patients for SREs and adverse outcomes.
Several studies have shown the impact of enzalutamide, abiraterone, and radium-223 both in prolonging time to SRE and in improving overall survival 6, 12-16. Despite these data, there remain significant gaps in identifying predictors of SRE in patients with mCRPC. In a previous study of a smaller cohort of men with mCRPC, we identified year of metastases, biopsy grade group, primary localized treatment versus none, and PSA doubling time (PSADT) as significant predictors for SREs6. Most notably, we identified bone pain at the time of bone mCRPC diagnosis as the strongest predictor of SREs6. Other studies have identified grade group and number of bone lesions at diagnosis as significant risk factors for developing SREs17, 18. Given the impact of SREs on morbidity and mortality, the objective of this study was to perform one of the largest retrospective cohort studies in men with mCRPC with longer follow-up in order to identify predictors for SRE and the impact of these predictors on all-cause mortality. We hypothesized that our current patient population would yield comparable results to our prior study and confirm the influence of bone pain, biopsy grade group, and the number of bone metastases on increased risk for SREs. Given the close relationship between SRE development and poorer survival outcomes, we also hypothesized that bone pain, grade group, and the number of bone metastases would be significant predictors of all-cause mortality.
Patients and methods
Data Source
This study was approved by the Institutional Review Board at the Durham VA Medical Center. We queried the Shared Equal Access Regional Cancer Hospital (SEARCH) database which collects information on patients with prostate cancer treated with a variety of primary treatment types from eight Veterans Affairs Medical Centers (VAMCs) across the nation, including Durham, NC; Asheville, NC; Los Angeles, CA; Palo Alto, CA; San Diego, CA; San Francisco, CA; Augusta, GA; and Portland, OR19. Patient data included demographics, diagnostic results, patient tumor characteristics, and long-term outcomes.
Ascertainment of Study Cohort
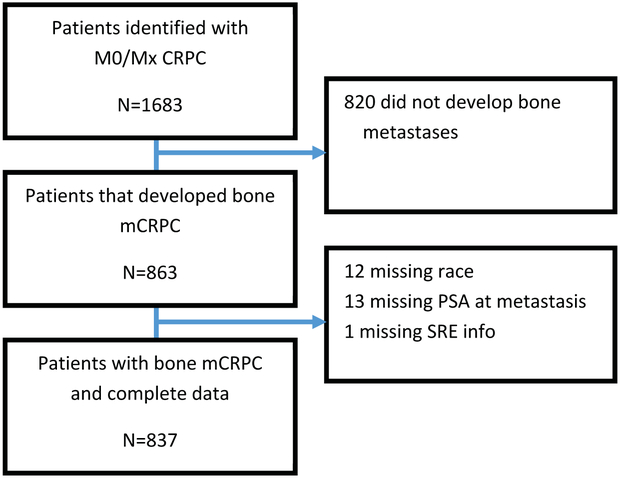
From the SEARCH database, we identified 1,683 men with non-metastatic CRPC. Of this population, 820 (49%) men did not develop bone metastases and were excluded. We identified 863 (51%) men with non-metastatic CRPC who developed bone metastases after CRPC diagnosis. We excluded 12 patients without race/ethnicity data, 13 patients without PSA values at mCRPC diagnosis, and 1 patient without SRE information, resulting in a final cohort of 837 patients (Figure 1).
Figure 1: Patient consort diagram.
Acronyms: M0/Mx = non-metastatic; CRPC = castration-resistant prostate cancer; mCRPC = metastatic castration-resistant prostate cancer; PSA = prostate-specific antigen; SRE = skeletal related event
Identification of Bone Metastases and CRPC
Adhering to the Prostate Cancer Working Group 2 criteria, we determined CRPC status as a ≥25% increase in PSA and an absolute increase of ≥2 ng/mL above the PSA nadir while castrate20. Castration was defined as testosterone values <50 ng/dl, the occurrence of a bilateral orchiectomy, or continuous receipt of a luteinizing hormone-release hormone agonist or antagonist6, 20. After identifying CRPC, we then individually reviewed imaging reports and progress notes to identify prostate cancer metastases to the bone, lungs, liver, non-pelvic lymph nodes (≥2 cm), and other visceral tissues. Patients who developed metastases before the date of CRPC were excluded in order to create a more homogeneous population of men newly presenting with mCRPC. To ensure accuracy of data collection, we randomly selected 10% of all collected data for secondary review.
Study Covariates
Through the SEARCH database, we abstracted patient characteristics including age, race (Black, White, Asian/Pacific Islander, American Indian/Alaska Native, Other, Unknown), ethnicity (Hispanic/Latino, Not Hispanic/Latino, Unknown), and treatment center. We collected grade group from the pathological report of the first positive biopsy. From the Veterans Affairs electronic medical record system, we abstracted primary localized treatment (none, radical prostatectomy, or radiation) and bone pain within two months before or after the development of bone metastases. Given insufficient medical record documentation, we were unable to determine location or intensity of pain. We identified the number of bone metastases at the time of bone metastases diagnosis by abstracting the number of lesions documented in radiology reports. PSA at metastases was characterized as the most recent PSA within one year prior to metastatic diagnosis. Patients were considered to have concomitant visceral (liver, lungs, other soft issue) or lymph node metastasis at baseline if the date of diagnosis was within one month before or after bone metastasis diagnosis.
Long-Term Outcomes
To examine predictors of SRE in men with bone mCRPC, we followed patients until date of death or date of last follow-up. SREs were defined as a pathologic fracture, spinal cord compression, radiation to the bone, or surgery to the bone7. Using imaging reports and medical records, we abstracted the first incidence of an SRE after the development of bone metastases. In determining patients’ cause of death, we characterized prostate cancer deaths as patients with progressive metastatic, castration resistant prostate cancer who died without another clear cause of death6. Any ambiguous cause of deaths were reviewed by the lead study physician (SJF).
Statistical analysis
PSADT was calculated as log(2) divided by the slope of the linear regression of log(PSA) over time in months. We required at least two PSAs over at least 90 days. We used all PSAs two years prior to date of initial metastasis and up to the date of metastasis, but starting after the date of CRPC diagnosis if that was within two years of metastasis diagnosis.
Patient demographics and disease characteristics were summarized across all patients. The date of first bone metastasis was treated as time zero for all analyses. Kaplan-Meier curves were created to show time to SRE and time to all-cause mortality. Cox proportional hazards models were used to examine predictors of SRE and all-cause mortality. For each outcome, a backwards stepwise selection process using criteria of α=0.1 for entry and α=0.2 for removal was used to select predictors. We chose a threshold value of α=0.2 in order to not exclude variables that are important but did not reach significance. Candidate covariates included race (black, non-black), age at metastases (continuous), year of metastases (2000-2003, 2004-2007, 2008-2012, 2013-2017), treatment center (categorical), biopsy grade group (1, 2-3, 4-5, unknown), primary localized treatment (none, radical prostatectomy ± radiation, radiation alone), number of bone metastases (1, 2, 3-9, ≥10, unknown), bone pain (yes, no, unknown), PSA at bone metastasis (continuous, log-transformed), PSADT (<9 months, ≥9 months, not calculable), presence of lymph node metastasis (yes, no), presence of visceral metastasis (yes, no), months from ADT to CRPC (continuous), and months from CRPC to bone metastasis (continuous). All statistical tests were 2-sided, and all analyses were performed using Stata 15.1 (StataCorp LLC, College Station, TX). Statistical significance was defined as p < 0.05.
Results
Patient Characteristics
Of 837 patients included in the analysis, 28% were black (Table 1). Median (IQR) age at metastasis was 76 (69-83). There were 328 men (39%) who received no primary localized treatment, 243 (29%) received radical prostatectomy ± radiation, and 266 (32%) received radiation alone. The number of patients with 1, 2, 3-9, and ≥10 bone metastases was 212 (25%), 131 (16%), 306 (37%), and 180 (22%), respectively. At the time of initial bone metastasis diagnosis, 109 (13%) men also had non-pelvic lymph node metastases and 44 (5%) men had visceral metastases. Median (IQR) PSA at metastasis was 31.5 (12.0-102.6), and 442 (53%) had PSADT <9 months. For 67% of patients, the PSA before metastasis was within 1 month of metastasis, 92% were within 3 months, and 98% were within 6 months. There were 362 (43%) men who reported bone pain at the time of metastasis diagnosis.
Table 1.
Patient characteristics at the time of metastasis
| Characteristic | Total (N=837) |
|---|---|
| Age at metastasis | |
| Median (IQR) | 76 (69,83) |
| Race | |
| Non-black | 605 (72%) |
| Black | 232 (28%) |
| Treatment center | |
| West LA | 154 (18%) |
| Palo Alto | 94 (11%) |
| San Francisco | 71 (8%) |
| Augusta | 68 (8%) |
| Durham | 157 (19%) |
| San Diego | 149 (18%) |
| Asheville | 46 (5%) |
| Portland | 98 (12%) |
| Biopsy grade group | |
| 1 | 109 (13%) |
| 2-3 | 171 (20%) |
| 4-5 | 249 (30%) |
| Unknown | 308 (37%) |
| Primary localized treatment | |
| None | 328 (39%) |
| RP ± XRT | 243 (29%) |
| XRT alone | 266 (32%) |
| Number of bone metastases | |
| 1 | 212 (25%) |
| 2 | 131 (16%) |
| 3-9 | 306 (37%) |
| ≥10 | 180 (22%) |
| Unknown | 8 (1%) |
| Lymph node metastasis | 109 (13%) |
| Visceral metastasis | 44 (5%) |
| PSA at metastasis | |
| Median (IQR) | 31.5 (12.0, 102.6) |
| PSADT at metastasis | |
| ≥9 months | 246 (29%) |
| <9 months | 442 (53%) |
| Unknown | 149 (18%) |
| Months from ADT to CRPC | |
| Median (IQR) | 42.0 (18.9, 74.4) |
| Months from CRPC to metastasis | |
| Median (IQR) | 15.1 (6.1, 31.7) |
| Bone pain | |
| No | 341 (41%) |
| Yes | 362 (43%) |
| Unknown | 134 (16%) |
| Follow-up | |
| Median (IQR) | 25.9 (12.0, 46.4) |
IQR: interquartile range
Follow-up
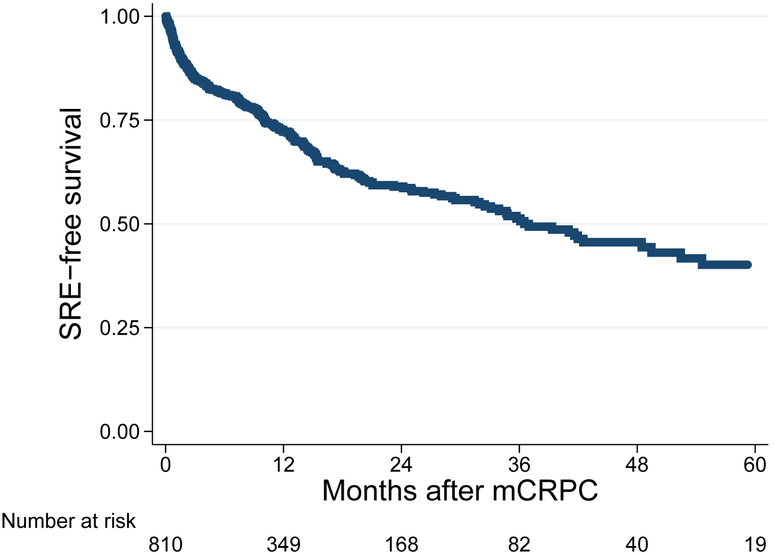
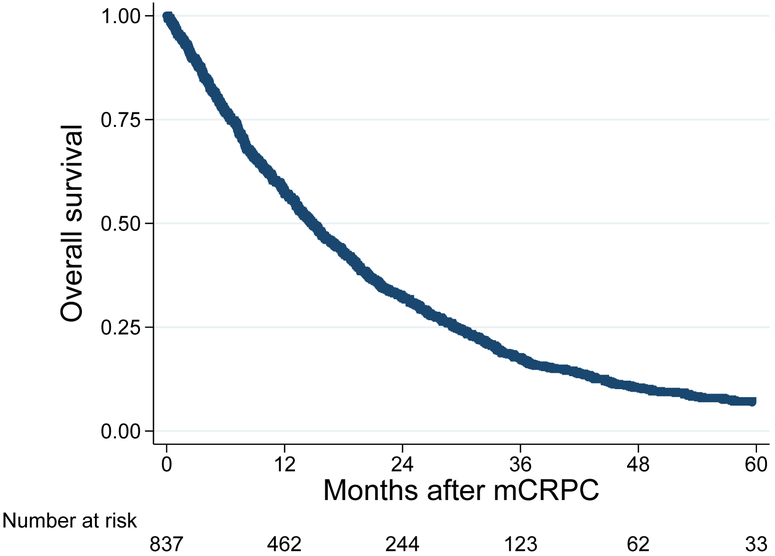
Median follow-up (IQR) was 26 months (12-46) among men who had not died and 14 months (6-27) among all patients. During follow-up, 287 patients developed an SRE and 740 men died. SREs were comprised of 29 pathological fractures, 236 radiation to the bone, 22 spinal cord compressions, and 4 surgeries to the bone. Median time to SRE was 36.5 months (Figure 2). Five-year SRE-free survival was 40%. Median time to all-cause mortality was 14.9 months (Figure 3). Five-year overall survival was 7%.
Figure 2: Kaplan Meier curve for time from bone metastasis to SRE.
Acronyms: SRE = skeletal related event; mCRPC = metastatic castration-resistant prostate cancer
Figure 3: Kaplan Meier curve for time from bone metastasis to mortality.
Acronym: mCRPC = metastatic castration-resistant prostate cancer
Predictors of SREs
On model selection for the SRE outcome, year of metastasis, bone pain, PSA at metastasis, time from ADT to CRPC, time from CRPC to metastases, presence of lymph node metastases, and presence of visceral metastases entered the model (Table 2). Specifically, bone pain (HR 2.96, 95% CI 2.25-3.89) and presence of visceral metastasis (HR 1.91, 95%CI 1.18-3.09) were associated with increased risk of SRE, while longer time from CRPC to metastasis (HR 0.92, 95%CI 0.85-0.99) and longer time from ADT to CRPC (HR 0.94, 95%CI 0.91-0.98) were associated with decreased risk of SRE.
Table 2:
Independent predictors of SRE among mCRPC patients
| Model Selection* | |||
|---|---|---|---|
| Variables | HR | 95% CI | P |
| Treatment center | 0.17 | ||
| Center 1 | Ref. | ||
| Center 2 | 0.62 | 0.37-1.02 | |
| Center 3 | 0.75 | 0.45-1.26 | |
| Center 4 | 0.84 | 0.48-1.47 | |
| Center 5 | 1.16 | 0.80-1.70 | |
| Center 6 | 1.07 | 0.73-1.58 | |
| Center 7 | 0.76 | 0.43-1.34 | |
| Center 8 | 0.81 | 0.52-1.27 | |
| Bone pain | <0.001 | ||
| No | Ref. | ||
| Yes | 3.00 | 2.27-3.96 | |
| Unknown | 1.49 | 0.98-2.25 | |
| PSA at metastases | 1.07 | 0.98-1.17 | 0.13 |
| Years from ADT to CRPC | 0.94 | 0.91-0.98 | 0.004 |
| Years from CRPC to mets | 0.91 | 0.84-0.98 | 0.010 |
| Lymph node metastasis | 0.054 | ||
| No | Ref. | ||
| Yes | 0.69 | 0.48-1.01 | |
| Visceral metastasis | 0.013 | ||
| No | Ref. | ||
| Yes | 1.83 | 1.13-2.96 | |
ADT: androgen deprivation therapy; CI: confidence interval; CRPC: castration-resistant prostate cancer; HR: hazard ratio; SRE: skeletal-related event
Backwards stepwise selection with α=0.1 for entry and α=0.2 for removal
Predictors of All-Cause Mortality
On model selection for the mortality outcome, age, year, primary localized treatment, number of bone metastases, bone pain, PSA, PSADT, time from ADT to CRPC, and presence of visceral metastasis entered the model (Table 3). Specifically, older age (HR 1.03, 95%CI 1.02-1.04), greater number of bone metastases (HR 1.37-2.17), bone pain (HR 1.44, 95%CI 1.23-1.70), higher PSA at metastasis (HR 1.21, 95%CI 1.14-1.28), and the presence of visceral metastasis (HR 1.72, 95%CI 1.23-2.39) were associated with increased risk of mortality, while receiving primary localized treatment (specifically radical prostatectomy compared to no treatment) (HR 0.73, 95%CI 0.61-0.89) and longer time from ADT to CRPC (HR 0.97, 95%CI 0.94-0.99) were associated with decreased risk of mortality. While PSADT as a whole (including the unknown category) was not associated with mortality (p=0.11), PSADT <9 months was associated with increased risk of mortality compared to PSADT ≥9 months (HR 1.21, 95%CI 1.00-1.45).
Table 3:
Independent predictors of mortality among mCRPC patients
| Model Selection* | |||
|---|---|---|---|
| Variables | HR | 95% CI | P |
| Age at metastasis | 1.02 | 1.02-1.04 | <0.001 |
| Year of metastasis | 0.98 | 0.96-0.99 | 0.010 |
| Primary localized treatment | 0.005 | ||
| None | Ref. | ||
| RP ± Radiation | 0.74 | 0.61-0.89 | |
| Radiation Alone | 0.95 | 0.80-1.14 | |
| Number of bone metastases | <0.001 | ||
| 1 | Ref. | ||
| 2 | 1.36 | 1.07-1.72 | |
| 3-9 | 1.40 | 1.15-1.71 | |
| ≥10 | 2.18 | 1.73-2.75 | |
| Unknown | 2.94 | 1.43-6.04 | |
| Bone pain | <0.001 | ||
| No | Ref. | ||
| Yes | 1.44 | 1.22-1.70 | |
| Unknown | 1.37 | 1.10-1.71 | |
| PSA at metastasis (ng/mL) | 1.20 | 1.14-1.27 | <0.001 |
| PSADT at metastasis (mo.) | 0.10 | ||
| ≥9 | Ref. | ||
| <9 | 1.26 | 1.05-1.50 | |
| Unknown | 1.16 | 0.93-1.46 | |
| Years from ADT to CRPC | 0.97 | 0.94-0.99 | 0.004 |
| Visceral metastasis | 0.001 | ||
| No | Ref. | ||
| Yes | 1.72 | 1.23-2.39 | |
ADT: androgen deprivation therapy; CI: confidence interval; CRPC: castration-resistant prostate cancer; HR: hazard ratio; SRE: skeletal-related event.
Backwards stepwise selection with α=0.1 for entry and α=0.2 for removal
Discussion
Approximately 90% of men with mCRPC develop bone metastases and, consequently, are at a risk for developing an SRE5-7. In this study, a third of patients with bone mCRPC developed at least one SRE, consistent with prior clinical trials of men on SRE prevention clinical trials7, 21. We found that bone pain, visceral metastases at time of bone metastasis diagnosis, and a shorter time interval between the start of ADT and CRPC diagnosis were significant risk factors for SRE development. Moreover, the number of bone metastases, bone pain, PSA value at metastases, and visceral metastases at time of bone metastases diagnosis were identified as risk factors for all-cause mortality all-cause mortality.
Our study has several important findings. First, the presence of bone pain at time of bone mCRPC diagnosis increased the risk for an SRE nearly three-fold compared to the absence of bone pain. This confirms our previous study with a smaller cohort of men with mCPRC, where we identified bone pain as the strongest predictor of SREs 6. Moreover, another study of 86 patients compared SRE development with bone pain, where bone pain was self-reported on a scale from 0 to 19. This study showed that the percentage of SREs was nearly twice as high in patients with a pain score of at least 5 compared with patients with a pain score of less than 522. Similarly, a study among breast cancer patients noted comparable results, demonstrating that patients with bone pain had a higher number of SREs and worse survival rates than patients without pain23. It is important to note that nearly 83% (236 out of 287) of patients that developed a SRE in our study underwent radiation to the bone. The association between bone pain and increased risk of SRE may be attributed to the palliative use of radiation for painful bony metastases24, 25. Long-term use of ADT is known to change bone mineral density and subsequently, may correlate with an increase in patients’ risk for fracture and bone metastases24, 25. The 2011 European Association of Urology (EAU) guidelines suggest external beam radiation therapy as a means for managing pain associated with osseous metastatic lesions26. Interestingly, other trials examining the impact of zolendronic acid on SREs did not show a significant correlation between bone pain and disease progression or SRE development7, 21. However, these prior studies did not look at time to SRE but merely total number of patients who developed SRE. As such, the results are not directly comparable. Given the limited number of patients who experienced SREs beyond radiation to the bone, our findings warrant further investigation into the relationship between bone pain and other SREs such as spinal cord compression, pathological fracture, and surgery to the bone.
Second, patients with visceral metastases within one month before or after bone metastasis diagnosis were more likely to develop an SRE than those without visceral metastases (HR 1.91, 95%CI 1.18-3.09). A study using SEER (Surveillance, Epidemiology, and End Results) Medicare-linked data examined the risk of SRE development depending on metastatic site17 . In a cohort of metastatic men, they found that patients with metastases in bone and lymph nodes, bone and “other” sites (including visceral metastases) ± lymph nodes, and “other” sites only (including visceral metastases) were as likely as patients with bone metastases only to develop SREs17. It is important to note that in our study, only 44 (5%) men within our mCRPC cohort had visceral metastases at the time of their bone metastasis diagnosis, though despite these small numbers, we saw a significant association.
Third, patients with a shorter duration of time between ADT start date and CRPC diagnosis were at a greater risk for developing SREs. This relationship could be reflective of disease aggressiveness. Both SREs and shorter time from prostate cancer diagnosis to CRPC diagnosis have been associated with markers for disease severity such as increased mortality and poorer survival outcomes10, 27, 28. Our previous study did not reveal similar findings. In our prior study, we found that the number of months between ADT start date and CRPC did not impact the risk for developing a SRE (HR 1.00, 95%CI 0.99-1.01, p=0.948)6. It is important to note that both studies have comparable median months from ADT to CRPC (42.0 months in our present study and 40.9 months in our previous study). The difference may come from having a larger cohort in the current study. Moving forward, it is important to consider the biological factors that may be contributing to this timeframe. For example, in a retrospective study of 122 men where 62% had at least four bone metastases, Sharma et al. found that elevated levels of IL-8, TNF-α, and MCP-1 were associated with shorter time intervals between the start of ADT and progression to CRPC29. It is also imperative to consider implementing clinical practices that could slow progression to CRPC, such as treating metastatic, hormone sensitive patients with ADT and docetaxel or ADT and abiraterone concomitantly30-32.
Fourth, we identified several predictors for mortality including number of bone metastases, bone pain, PSADT, and the presence of visceral metastases at time of mCRPC diagnosis. The strongest predictor for all-cause mortality was number of bone metastatic lesions. Patients with more than ten metastatic bone lesions at time of mCRPC diagnosis were at a high risk for all-cause mortality (HR 2.17). Prior studies have shown similar results. Tait et al. studied skeletal metastases in CRPC patients. Patients with 5-20 metastases had a median overall survival value of 22.1 months whereas patients with ≥20 metastases had a median overall survival time of 13.3 months33. Interestingly, we found that all-cause mortality was influenced by primary therapy type. A regimen of radiation and radical prostatectomy (RP) had a protective effect on patients’ all-cause mortality as compared to no localized therapy at all. 328 (39%) of the men within our cohort did not receive primary localized therapy but immediately began on systemic therapy. It is important to note, however, that immediate hormonal therapy is often the treatment choice for patients with advanced asymptomatic disease who do not pursue local therapy26, 34. As such, whether local treatment truly impacts the natural progress of the disease or is merely a marker of men who had lower volume disease is unknown.
Overall, it is important to consider treating men with bone pain or visceral metastases at time of metastatic diagnosis with agents known for decreasing risk of developing SREs, The NCCN guidelines recommend agents such as bisphosphonates or denosumab for men on androgen deprivation with a high risk for SRE35. Clinical trials have shown the efficacy of agents such as zolendronic acid, denosumab, enzalutamide, abiraterone, and radium-223 in reducing SREs for men with mCRPC7, 12-16, 32, 36. Studies have also shown the impact of enzalutamide, abiraterone, and radium-223 both in prolonging time to SRE and in improving overall survival6, 12-16.
Our findings need to be interpreted in the context of our study design. Our cohort consisted of men with mCRPC treated at 8 hospitals of the equal-access, equal payer Veterans Affairs (VA) health care system. Although this is an improvement in generalizability compared to our previous study that had data from only 2 VA hospitals, the retrospective nature of the study with inherent selection bias remains a limitation6. As such, treatment decisions and radiology imaging were conducted at the discretion of the treating physician. Additionally, given the study’s retrospective nature, we cannot deduce causality or, for that matter, reverse causality. Though the majority of SRE events were radiation to the bone, which would occur after pain (i.e. the pain caused the need to treat leading to the SRE), it is possible that for some men there was reverse causality in that the SRE (i.e. spinal cord compression or pathological fracture) occurred first but was not yet diagnosed and the pain was the symptom of the SRE. Moreover, we examined a cohort of men who initially presented with non-metastatic CRPC and later developed mCRPC to create a more homogenous group of men. Unfortunately, this serves to limit the generalizability of our results as they may not apply to men who presented with metastatic hormone-sensitive disease and then progressed to mCRPC. Furthermore, although we had access to complete medical records for abstracting bone pain and determining SRE development and risk, we did not have a standardized method for documenting bone pain presence and severity. As such, there may be some uncertainty in this variable. However, as any potential inaccuracies in data tend to bias the results to the null, our study may have underestimated the true strength of the association between bone pain and SRE. Moreover, we did not analyze the percentage of patients on bone protective agents or address the impact of opiate use. We did not have the data to address the availability, approval or usage of these agents. This is a limitation as our data spans a time at which these agents were accessible to patients. Future studies are needed to address the impact of bone protective agents and opiates on SRE development. Finally, our study only examined time to first SRE. Future studies are needed to assess multiple SREs over time. A strength of our study is in its sample size which allowed us to characterize multiple end points for our multivariate analysis. Moreover, 34% of patients in our present study developed an SRE, which is a rate that is comparable to previous retrospective and phase 3 studies6, 21.
Conclusions
In this cohort of mCRPC men with bone metastatic disease, we identified bone pain as the strongest predictor for SRE development and the number of bone metastases at mCRPC diagnosis as the strongest predictor for all-cause mortality. If validated in future studies, these factors may be used to risk stratify patients. Patients with bone pain or more than ten bone metastatic lesions should receive preventative interventions to preserve overall quality of life and prolong survival.
Acknowledgements:
This study was conducted with the support of Bayer Pharmaceuticals (SJF).
Funding: Support for this study was provided by the NIH/NCI under Award Number R01CA231219 and NIH K24 CA160653
Footnotes
Conflict of Interest: None.
References
- 1.Jemal A, Fedewa SA, Ma J, et al. Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. Jama. 2015;314: 2054–2061. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68: 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004-2013). Prostate Cancer Prostatic Dis. 2016;19: 395–397. [DOI] [PubMed] [Google Scholar]
- 4.Lowrance WT, Murad MH, Oh WK, Jarrard DF, Resnick MJ, Cookson MS. Castration-Resistant Prostate Cancer: AUA Guideline Amendment 2018. J Urol. 2018. [DOI] [PubMed] [Google Scholar]
- 5.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31: 578–583. [DOI] [PubMed] [Google Scholar]
- 6.Klaassen Z, Howard LE, de Hoedt A, et al. Factors predicting skeletal-related events in patients with bone metastatic castration-resistant prostate cancer. Cancer. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 8.McDougall JA, Bansal A, Goulart BH, et al. The Clinical and Economic Impacts of Skeletal-Related Events Among Medicare Enrollees With Prostate Cancer Metastatic to Bone. Oncologist. 2016;21: 320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roghmann F, Antczak C, McKay RR, et al. The burden of skeletal-related events in patients with prostate cancer and bone metastasis. Urol Oncol. 2015;33: 17.e19–17.e18. [DOI] [PubMed] [Google Scholar]
- 10.Howard LE, De Hoedt AM, Aronson WJ, et al. Do skeletal-related events predict overall survival in men with metastatic castration-resistant prostate cancer? Prostate Cancer Prostatic Dis. 2016;19: 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resnick MJ, Penson DF. Quality of life with advanced metastatic prostate cancer. Urol Clin North Am. 2012;39: 505–515. [DOI] [PubMed] [Google Scholar]
- 12.Graff JN, Beer TM. Reducing Skeletal-Related Events in Metastatic Castration-Resistant Prostate Cancer. Oncology (Williston Park). 2015;29: 416–423. [PubMed] [Google Scholar]
- 13.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 14.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15: 738–746. [DOI] [PubMed] [Google Scholar]
- 17.Hussain A, Aly A, Daniel Mullins C, Qian Y, Arellano J, Onukwugha E. Risk of skeletal related events among elderly prostate cancer patients by site of metastasis at diagnosis. Cancer Med. 2016;5: 3300–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchekmedyian NS, Chen YM, Saad F. Disease progression increases the risk of skeletal-related events in patients with bone metastases from castration-resistant prostate cancer, lung cancer, or other solid tumors. Cancer Invest. 2010;28: 849–855. [DOI] [PubMed] [Google Scholar]
- 19.Vidal AC, Howard LE, Sun SX, et al. Obesity and prostate cancer-specific mortality after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Prostate Cancer Prostatic Dis. 2017;20: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saad F, Eastham J. Zoledronic Acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology. 2010;76: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 22.Berruti A, Tucci M, Mosca A, et al. Predictive factors for skeletal complications in hormone-refractory prostate cancer patients with metastatic bone disease. Br J Cancer. 2005;93: 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koizumi M, Yoshimoto M, Kasumi F, Iwase T, Ogata E. Post-operative breast cancer patients diagnosed with skeletal metastasis without bone pain had fewer skeletal-related events and deaths than those with bone pain. BMC Cancer. 2010;10: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vignani F, Bertaglia V, Buttigliero C, Tucci M, Scagliotti GV, Di Maio M. Skeletal metastases and impact of anticancer and bone-targeted agents in patients with castration-resistant prostate cancer. Cancer Treat Rev. 2016;44: 61–73. [DOI] [PubMed] [Google Scholar]
- 25.Weinfurt KP, Li Y, Castel LD, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005;16: 579–584. [DOI] [PubMed] [Google Scholar]
- 26.Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2011;59: 572–583. [DOI] [PubMed] [Google Scholar]
- 27.Bournakis E, Efstathiou E, Varkaris A, et al. Time to castration resistance is an independent predictor of castration-resistant prostate cancer survival. Anticancer Res. 2011;31: 1475–1482. [PubMed] [Google Scholar]
- 28.Nakabayashi M, Hayes J, Taplin ME, et al. Clinical predictors of survival in men with castration-resistant prostate cancer: evidence that Gleason score 6 cancer can evolve to lethal disease. Cancer. 2013;119: 2990–2998. [DOI] [PubMed] [Google Scholar]
- 29.Sharma J, Gray KP, Harshman LC, et al. Elevated IL-8, TNF-alpha, and MCP-1 in men with metastatic prostate cancer starting androgen-deprivation therapy (ADT) are associated with shorter time to castration-resistance and overall survival. Prostate. 2014;74: 820–828. [DOI] [PubMed] [Google Scholar]
- 30.Bernard B, Sweeney CJ. Management of metastatic hormone-sensitive prostate cancer. Curr Urol Rep. 2015;16: 14. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney CJ, Chen Y-H, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. New England Journal of Medicine. 2015;373: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. New England Journal of Medicine. 2017;377: 352–360. [DOI] [PubMed] [Google Scholar]
- 33.Tait C, Moore D, Hodgson C, et al. Quantification of skeletal metastases in castrate-resistant prostate cancer predicts progression-free and overall survival. BJU Int. 2014;114: E70–e73. [DOI] [PubMed] [Google Scholar]
- 34.Patel DN, Jha S, Howard LE, et al. Impact of prior local therapy on overall survival in men with metastatic castration-resistant prostate cancer: Results from Shared Equal Access Regional Cancer Hospital. Int J Urol. 2018;25: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomella LG, Petrylak DP, Shayegan B. Current management of advanced and castration resistant prostate cancer. Can J Urol. 2014;21: 1–6. [PubMed] [Google Scholar]
- 36.Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic Prostate Cancer and the Bone: Significance and Therapeutic Options. Eur Urol. 2015;68: 850–858. [DOI] [PubMed] [Google Scholar]