Abstract
Objectives
The aim of this study was to describe the cohort of patients who have been treated with Denosumab as neoadjuvant therapy prior to surgery for aggressive giant cell tumor of bone in the extremities, to evaluate the radiological responses to Denosumab comparing Choi criteria and a newly described computerized tomography (CT) classification, and to evaluate the risk of local recurrence after intralesional curettage or radical excision.
Methods
We retrospectively evaluated 36 patients (20 females and 16 males; mean age at diagnosis 36 years (range, 18–64)) treated with neoadjuvant Denosumab therapy prior to surgery for aggressive giant cell tumor of bone in the extremities. The radiological responses to Denosumab treatment were analyzed on the preoperative images after the neoadjuvant course with the Choi criteria and with a newly proposed classification based on CT. All these images were independently reviewed by two of the researchers. Surgical intervention methods were noted and local recurrence rates were evaluated. The correlation between radiological response amount and local recurrence were analyzed for both Choi criteria and the new CT classification.
Results
Denosumab was administered for a mean of 21 weeks (range 7–133). Five patients also had a short postoperative course. According to Choi criteria there was a radiological response in 32 patients (89%), while the new CT classification identified responses in all the 36 patients (100%). The identification of changes after 7 weeks of treatment was higher using the CT classification compared to Choi criteria (p = 0.043 vs p = 0.462). The surgical interventions after Denosumab comprised curettage in 29 patients (74%) and resection in 7 (26%). Local recurrence was higher in patients managed with intralesional curettage than in those treated with en bloc resection (55.1% vs 0%, p < 0.001). At last follow up 19 patients (53%) required en bloc resections. Good responders to Denosumab (type 2C) had lower risk of local recurrence (p = 0.047) after either resection or curettage.
Conclusion
The new CT classification evaluated more accurately the response to Denosumab. Our experience suggests that the requirement for radical bone resection remains high despite the use of Denosumab.
Level of evidence
Level IV, Therapeutic Study.
Keywords: Giant cell tumour, Curettage, Resection, Denosumab, Arthritis
Introduction
Giant-cell tumor of bone (GCTB) is a benign but aggressive skeletal neoplasm that most commonly affects young adults. The disease characteristically presents as a meta-epiphyseal, lytic lesion and it is most often located in the distal femur, proximal tibia, and distal radius.1 The cortex of the involved bone is usually thinned, expanded, and sometimes breached with or without soft tissue extension. One of the main concerns in the management of these tumors is the high local recurrence (LR) rate (range 21–65%).2 Local recurrence is influenced by several factors including age of the patient, Campanacci grade, tumor location, soft tissue extension, pathological fracture, the type of treatment performed. Moreover, several molecular an genetic factors have been proposed as risk factors for LR.3, 4
Intralesional curettage, aims to preserve the bone structure but it is unfortunately associated with higher risk of LR.5 Therefore, several adjuvant treatments have been proposed to reduce the risk of recurrence, including phenol, liquid nitrogen and polymethylmethacrylate cement.1 On the other hand, radical bone resections with wide margins reduce LR rates, but they are associated with increased morbidity and possible functional impairment.6
During the last decade, a monoclonal antibody that inhibits RANKL called Denosumab has started to be considered as a new option for the treatment of locally advanced GCTB. Some of the mechanisms of action described are the suppression in the osteoclast differentiation, the increase of osteocalcin and the final differentiation of stromal cells into osteoblasts.7 Denosumab stimulate the reconstruction of a new peripheral osseous rim and the progressive ossification of the lesion after its administration. This effect switches the stage of the disease from aggressive to active or latent. Denosumab may therefore allow conservative surgery with bone and joint preserving surgery in patients with large tumors that would have been treated with radical resections in the past.8, 9 Denosumab has been reported to result in beneficial surgical downstaging10, 11, 12; however, the results were from patients who remained on denosumab or in whom it had been discontinued but with a short follow-up.10, 11, 12
Even though Denosumab downstages the GCTB, concerns have been raised for the reactivation of the disease after stopping the treatment.13 Moreover, there are still some doubts on which is the most appropriate surgery after Denosumab and the long-term results after intralesional curettage.14 Additionally, response to Denosumab therapy is generally evaluated with classification originally proposed to evaluate responses to chemotherapy in cancer, such as MD Anderson criteria, RECIST criteria, modified EORTC criteria and Choi criteria.11, 15 Denosumab cannot make GCT disappear but it makes the tumour calcify. Thus, it is not possible to have a “complete response” to therapy as described in previous classifications.
The aim of this report is to describe the cohort of patients who have been treated with Denosumab as neoadjuvant therapy and evaluate the radiological responses. In addition, we evaluated if the use of Denosumab can modify the evolution of the disease, the development of LR, and whether or not it allows us to perform conservative surgeries thereby maintaining the bone and joint structure in the long-term.
Patients and methods
At our Institution, 63 patients have been treated with Denosumab for aggressive Campanacci stage 2–3 GCTB from 2010 to 2016.
Adult patients (≥18 years) affected by Campanacci grade 2–3 GCTB with localization in the limbs, who underwent surgery after Denosumab treatment were included. All cases were histologically revised by experienced pathologist at our Institution.
Patients who received Denosumab to treat lung metastasis at diagnosis, patients who received Denosumab as a definitive treatment without any surgery and those included in the initial trials were excluded.10, 16 Also, patients who had known or suspected diagnosis of sarcoma, diagnosis of second malignancy within 5 years, who were lost to follow-up, who had incomplete medical records and who died for other reasons during treatment were excluded from the study.
We identified a total of 36 patients who received Denosumab as neoadjuvant therapy to surgery which were included in this retrospective analysis. All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. The independent ethics committee of our Institution (Istituto Ortopedico Rizzoli, Bologna) approved the study (File number: 0006276).
Denosumab (Xgeva® 120 mg) was given as a subcutaneous injection every 4 weeks (with loading doses on day 8 and 15 in cycle 1). Planned duration of neoadjuvant denosumab was 6–12 months, and the duration of adjuvant (postoperative) treatment was 6 months.
All patients underwent a routine dental exam before starting treatment, and were advised to take daily supplements containing 500–1000 mg calcium and 400–800 IU vitamin D.
Intralesional surgery (curettage) consisted of intralesional curettage, additional high-speed burring, followed by filling of the defect with cementing. No adjuvant intraoperative treatments were used.
Radical surgery was defined as a wide resection with or without reconstruction. The surgical procedures after Denosumab administration were considered initial surgery for analytical purposes.
The response to Denosumab treatment was evaluated on the preoperative images after the neoadjuvant course. All these images were independently reviewed by two of the researchers (AS, RM). The radiological responses to Denosumab were analyzed with the Choi criteria17 (Table 1) and with a newly proposed classification based on computerized tomography (CT) (see Fig. 1).
Table 1.
Choi criteria.
| Response | Definition |
|---|---|
| Complete response (CR) | Disappearance of all lesions |
| Partial response (PR) | A decrease in size of ≥10% or a decrease in tumour density ≥15% |
| Stable disease (SD) | Does not meet the criteria for CR, PR or PD. |
| Progression of disease (PD) | An increase in tumour size of ≥10% |
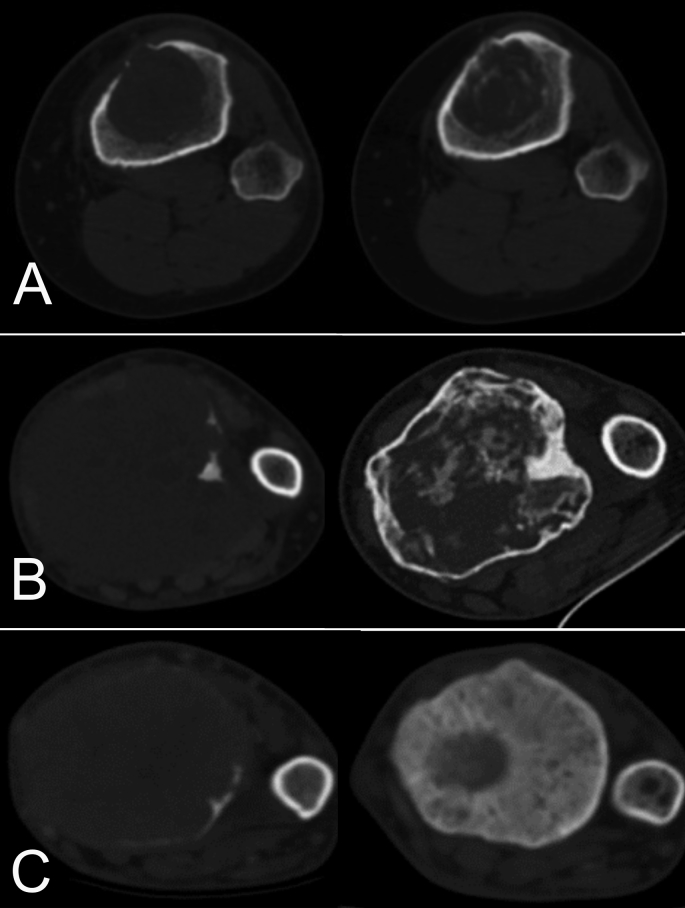
Fig. 1.
Computerized tomography (CT) classification to asses GCTB changes after Denosumab. On the left axial CT showing tumour at diagnosis, on the right after Denosumab treatment. A) Type 2A response: Same size, ossification of the shell and internal ossification <30%; B) Type 2B response: Same size, ossification of the shell and internal ossification 30–60%; C) Type 2C response: Reduce size, ossification of the shell and internal ossification >60%.
Choi criteria were evaluated according to the modifications both in size on different planes and in density (calcification) of the tumour on CT scan.
According to the new ct classification, a progression of the disease (Type 0 response) was defined when an increase ≥ 25% of the tumour was observed. We defined Stable disease (Type 1) those cases with an increase < 25% of GCT. Partial response (Type 2) was defined as a GCT stable in size and with ossification of the shell. It was sub classified according to the percentage of internal ossification (Type 2A: <30%; Type 2B: 30–60%; Type 2C: >60%).
The newly CT classification cut-offs were decided arbitrarily, and verified according to our data (Table 2).
Table 2.
Newly proposed classification based on computerized tomography to assess response to Denosumab treatment.
| Response | CT classification | Description |
|---|---|---|
| Progression | 0 | Increase ≥25% size of measurable lesions |
| Stable | 1 | <25% increase size of measurable lesions |
| Partial | 2A | Same size, ossification of the shell and internal ossification <30% |
| 2B | Same size, ossification of the shell and internal ossification 30–60% | |
| 2C | Reduce size, ossification of the shell and internal ossification >60% |
At latest follow-up patients were functionally evaluated with the Musculoskeletal Tumor Society (MSTS) score.18 Moreover, the presence of arthritis was evaluated according to the Kellgren–Lawrence classification on the last x-rays available and was considered positive when grades 2 (space narrowing), 3 (osteophytes) or 4 (bone deformity) were observed.19
Patients' characteristics are presented by frequencies and percentages for categorical variables, mean and range for continuous variables.
The differences between groups were compared by Mann–Whitney U test. Significance was set with P values < 0.05 in all statistical analyses, which were completed using the Statistical Package for Social Science (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
Results
In the cohort, there were 20 females (56%) and 16 males (44%). The mean age at diagnosis was 36 years (range, 18–64). Based on initial imaging, 25 (69%) tumors were classified as Campanacci grade 3 and 11 (31%) as Campanacci grade 2. Seventeen GCTB were located in the lower limb (6 in the distal femur, 8 in the proximal tibia, and three in the distal tibia), 19 in the upper limb (12 in the distal radius and 7 in the proximal humerus).
Denosumab was administered to 29 (81%) patients for newly diagnosed primary GCTB while 7 (19%) received it for locally recurrent GCTB.
Patients had surgery after a mean of 21 weeks of treatment (range 7–133). One patient (large, grade 3, distal radius GCT) received pre-operative course of Denosumab for 133 weeks as she originally refused surgery. Finally, she decided to go for surgery (resection and wrist arthrodesis) as she would like to become pregnant.
Five patients also had a postoperative adjuvant course of Denosumab for a mean of 23 weeks (range, 22–29 weeks).
Radiological response
According to Choi criteria, 4 patients were classified as stable disease and 32 presented a partial response before the surgical treatment. No correlation was observed between the length of the treatment and changes in the radiological response using Choi criteria (p = 0.462).
With the newly proposed CT classification, 8 patients presented a 2A response to Denosumab, 9 type 2B and 19 type 2C before the surgical intervention.
Both observer were concordant in the evaluation using the CT classification in 32 out of 36 cases with an inter observer reliability of 88.9%. In details, three out of 4 discordant cases were between type 2B and 2C and one between 2A and 2B.
Maximal response to Denosumab was observed in 33 out of 36 patients after 3 months of treatment. After that period, radiological response maintained a plateau.
Interventions and complications
The surgical treatments after Denosumab included curettage in 29 (81%) patients and resection in 7 (19%) patients. The type of treatment was chosen independently to the radiological response either with the Choi criteria (p = 0.546) or the CT classification (p = 0.451). The decision in terms of type of treatment was mainly affected by the location, intraarticular compromise and soft tissue extension.
Five (13%) patients presented complications related to Denosumab administration. Two patients developed hypocalcemia, two patients developed diffuse bone pain, and one patient presented a stress fracture in a different bone. The treatment had to be stopped at an early stage only in one of the patients presenting pain. The other complications were managed without further impact on the overall treatment.
Local recurrence
Mean follow-up was 35 months (range, 13–73 months).
The overall local recurrence rate was 16.7% (6 out of 36 patients) and the mean time to develop it was 17 months (range, 5–22 months). According to the presentation, a slightly higher but not significant LR rate was observed in patients with primary tumors compared with those presenting with recurrent GCTB (21% vs 13%, p = 0.457).
Based on the initial surgical treatment, 16 out of 29 patients managed with intralesional curettage presented LR while no recurrences were observed in those treated with en bloc resection (55.1% vs 0%, p < 0.001). The treatments for LRs included: conversion to endoprosthetic replacement in 5 patients, second curettage in 10 patients and long term Denosumab therapy in the other one patient.
Patients that presented radiological responses type 2C presented lower rates of local recurrence compared to all the other type of responses (p = 0.047). Nevertheless, no correlation was observed between the radiological responses and local recurrence when the Choi criteria were used (p = 0.284).
Final results
Excluding 5 patients who underwent resection for LR in the curettage group, radiographic signs of arthritis (Kellgren Lawrence Classification grades 2, 3 and 4) were observed in 7 out of the 24 patients (33%) that were treated with intralesional curettage after a mean of 23 months (range, 11–45) from initial surgery. All those patients were converted to en-bloc resections in the long term. At final follow up, the mean MSTS score did not differ between the group of patients initially treated with curettage (mean 23, range 22–29) and the radical treatment group (mean 22, range 17–29) (p = 0.674).
The final surgical procedures were intralesional curettage in 17 patients (47%) and en-bloc resections in 19 patients (53%). Comparing the initial and final procedures, 41% of the patients initially treated with intralesional curettage (12 out of 29) were converted to en-bloc resections either for local recurrence (5 patients) or arthritis (7 patients), while patients treated with resections had similar MSTS scores without instances of recurrence.
Discussion
The exact use of Denosumab in patients with Giant Cell Tumors of Bone is still a matter of controversy in orthopedic oncology. In patients with advanced or locally aggressive disease, the proposed benefit of Denosumab is to downstage the tumors, thereby allowing a more conservative surgery. Most of the published series have reported on the use of Denosumab for GCTB involving both axial and appendicular bones.16, 20 However, since surgery in centrally located GCTB has higher risks, with significant morbidity and important functional losses with respect to the extremities, the indications for the use of Denosumab may differ according to the location.
Another problem in the treatment with Denosumab is the lack of reliability between the clinical and radiological responses after the treatment. In most of the published series, Denosumab is shown to be clinically effective in GCTB16, 21, 22; however, the radiological outcome is generally evaluated using classifications that were set up to identify therapeutic responses in solid tumors.17, 23 These classifications do not consider the increase in the consolidation of the peripheral osseous rim and are more focused on the reduction in size which create difficulties in the determination of the actual response to Denosumab and therefore in the choice of the best treatment after the therapy.
Considering those problems, we described a new classification based on CT to assess bone changes after Denosumab in GCTB and then we compared that to Choi criteria.
We observed that the CT evaluation showed a better correlation with the radiological responses and higher sensitivity in the evaluation of the responses than Choi. Additionally, we observed a correlation between good radiological responses and lower local recurrence rates, which suggest that the evaluation of the radiological responses should be better characterized in order to identify higher-risk patients.
Most of the local recurrences observed in our cohort occurred after approximately 18 months after Denosumab stoppage, and the risk of local recurrence appeared to be mainly influenced by the type of initial surgical treatment performed. Traub et al22 already addressed this issue, but also reported good outcomes after Denosumab in conservatively treated GCTB. From our experience GCTB densely ossify following Denosumab treatment and therefore, curettage may be insufficient to remove the tumor completely.14, 21 In this series, intralesional curettage was associated with a higher rate of LR and a significant percentage of patients with X-rays signs of arthritis in the medium-term. Therefore, a very high number of patients required en-bloc resections as a final procedure.
For this reason, patients with severe articular compromise or deformity may benefit from Denosumab to consolidate the tumor, in order to perform en-bloc resection as first surgery. On the other hand, patients with a reasonable bone stock can have curettage and cementation or bone grafting after Denosumab in the attempt to preserve the joint. Nevertheless, the surgeon and patient have to be aware of the higher recurrence rate and possible development of arthritis when curettage is performed compared to resection.
The complications related to Denosumab in this series were similar to those reported in previous studies,10, 16 and most of them were managed without impact in the overall treatment. On the other hand, most of the responses to Denosumab were seen after approximately 5 months of treatment. We therefore feel that for patients with tumors compromising the articular surface, a short course of Denosumab (3–6 months) followed by wide resection will provide a safer outcome with fewer local recurrences and fewer side effects. On the other hand, if the joint surface is not involved and there are no deformities, Denosumab in a short course of 3 months followed by curettage might be advisable. Nevertheless, the surgeon has to be aware of the higher recurrence rate and possible development of arthritis. In addition to that, the follow-up in Denosumab patients, in particular in those treated with curettage, should be more detailed for at least two years after the end of the therapy, as recurrences might be seen later.
One of the limitations of this study is the retrospective analysis of the data, which might induce information bias. In addition, since the number of patients is small, we cannot draw any definitive conclusions regarding the differences in recurrence rates and complications between intralesional surgery and wide resection. Another weakness of this study is the relatively short follow up after Denosumab stoppage, which limits us to rule out possible relapses in the long term. In addition, no comparison was possible between the radiological results and pathological healing criteria.24 In addition, the study population is heterogeneous, as 11 large and grade 2 GCT with involvement of the subchondral bone received Denosumab, even though it is now well known that grade 2 Campanacci GCT can be managed without neoadjuvant course of Denosumab, This was due to the size and site of the tumors, in order to downstage these tumours. Moreover, pre-operative MSTS scores are lacking.
Due to that, the conclusions observed cannot be generalized. Additionally, we cannot evaluate any differences in the behavior of GCTB when Denosumab is administered for the primary tumor, compared to its use in patients presenting with locally recurrent GCTB. However, this is a mono-institutional series with all patients operated by two senior surgeons. This cohort describes accurately most of the current indications for the use of Denosumab in GCTB located in the extremities, and to the extent of our knowledge this is the only series based only on patients with GCTB located in appendicular bones.
Conclusions
The radiological classification based on CT images may be more accurate than Choi criteria in identifying more clearly early changes due to Denosumab therapy.
Most of the patients can be treated surgically when GCT is located in the extremities, and the use of Denosumab in short course seems to be appropriate. Surgery should be performed whenever possible and resection should be preferred in aggressive GCTB with articular compromise.
Conflicts of interest
All the authors declare they have no conflict of interests.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
References
- 1.Skubitz K.M. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507–518. doi: 10.1007/s11864-014-0289-1. [DOI] [PubMed] [Google Scholar]
- 2.van der Heijden L., Dijkstra P.D., van de Sande M.A. The clinical approach toward giant cell tumor of bone. Oncologist. 2014;19(5):550–561. doi: 10.1634/theoncologist.2013-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Y., Zhang J., Ding X. Prognosis of local recurrence in giant cell tumour of bone: what can we do? Radiol Med. 2017;122(7):505–519. doi: 10.1007/s11547-017-0746-6. [DOI] [PubMed] [Google Scholar]
- 4.Errani C., Ruggieri P., Asenzio M.A. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1–7. doi: 10.1016/j.ctrv.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Errani C., Tsukamoto S., Leone G. Denosumab may increase the risk of local recurrence in patients with giant-cell tumor of bone treated with curettage. J Bone Joint Surg Am. 2018;100(6):496–504. doi: 10.2106/JBJS.17.00057. [DOI] [PubMed] [Google Scholar]
- 6.Shehadeh A., Noveau J., Malawer M., Henshaw R. Late complications and survival of endoprosthetic reconstruction after resection of bone tumors. Clin Orthop Relat Res. 2010;468(11):2885–2895. doi: 10.1007/s11999-010-1454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosario M., Takeuchi A., Yamamoto N. Pathogenesis of osteosclerotic change following treatment with an antibody against rankl for giant cell tumour of the bone. Anticancer Res. 2017;37(2):749–754. doi: 10.21873/anticanres.11373. [DOI] [PubMed] [Google Scholar]
- 8.Branstetter D.G., Nelson S.D., Manivel J.C. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012;18(16):4415–4424. doi: 10.1158/1078-0432.CCR-12-0578. [DOI] [PubMed] [Google Scholar]
- 9.Lacey D.L., Boyle W.J., Simonet W.S. Bench to bedside: elucidation of the opg-rank-rankl pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11(5):401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 10.Chawla S., Henshaw R., Seeger L. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901–908. doi: 10.1016/S1470-2045(13)70277-8. [DOI] [PubMed] [Google Scholar]
- 11.Ueda T., Morioka H., Nishida Y. Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase ii trial. Ann Oncol. 2015;26(10):2149–2154. doi: 10.1093/annonc/mdv307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutkowski P., Ferrari S., Grimer R.J. Surgical downstaging in an open-label phase ii trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol. 2015;22(9):2860–2868. doi: 10.1245/s10434-015-4634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boye K., Jebsen N.L., Zaikova O. Denosumab in patients with giant-cell tumor of bone in Norway: results from a nationwide cohort. Acta Oncol. 2017;56(3):479–483. doi: 10.1080/0284186X.2016.1278305. [DOI] [PubMed] [Google Scholar]
- 14.Gaston C.L., Grimer R.J., Parry M. Current status and unanswered questions on the use of denosumab in giant cell tumor of bone. Clin Sarcoma Res. 2016;6(1):15. doi: 10.1186/s13569-016-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engellau J., Seeger L., Grimer R. Assessment of denosumab treatment effects and imaging response in patients with giant cell tumor of bone. World J Surg Oncol. 2018;16(1):191. doi: 10.1186/s12957-018-1478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmerini E., Chawla N.S., Ferrari S. Denosumab in advanced/unresectable giant-cell tumour of bone (Gctb): for how long? Eur J Cancer. 2017;76:118–124. doi: 10.1016/j.ejca.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Choi H., Charnsangavej C., Faria S.C. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25(13):1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 18.Enneking W.F., Dunham W.K. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60(6):731–746. [PubMed] [Google Scholar]
- 19.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rekhi B., Verma V., Gulia A. Clinicopathological features of a series of 27 cases of post-denosumab treated giant cell tumors of bones: a single institutional experience at a tertiary cancer referral centre, India. Pathol Oncol Res. 2017;23(1):157–164. doi: 10.1007/s12253-016-0123-0. [DOI] [PubMed] [Google Scholar]
- 21.Muller D.A., Beltrami G., Scoccianti G., Campanacci D.A., Franchi A., Capanna R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol. 2016;14(1):281. doi: 10.1186/s12957-016-1034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traub F., Singh J., Dickson B.C. Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur J Cancer. 2016;59:1–12. doi: 10.1016/j.ejca.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Costelloe C.M., Chuang H.H., Madewell J.E., Ueno N.T. Cancer response criteria and bone metastases: recist 1.1, Mda and percist. J Cancer. 2010;1:80–92. doi: 10.7150/jca.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deveci M.A., Paydas S., Gonlusen G., Ozkan C., Bicer O.S., Tekin M. Clinical and pathological results of denosumab treatment for giant cell tumors of bone: prospective study of 14 cases. Acta Orthop Traumatol Turc. 2017;51(1):1–6. doi: 10.1016/j.aott.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]