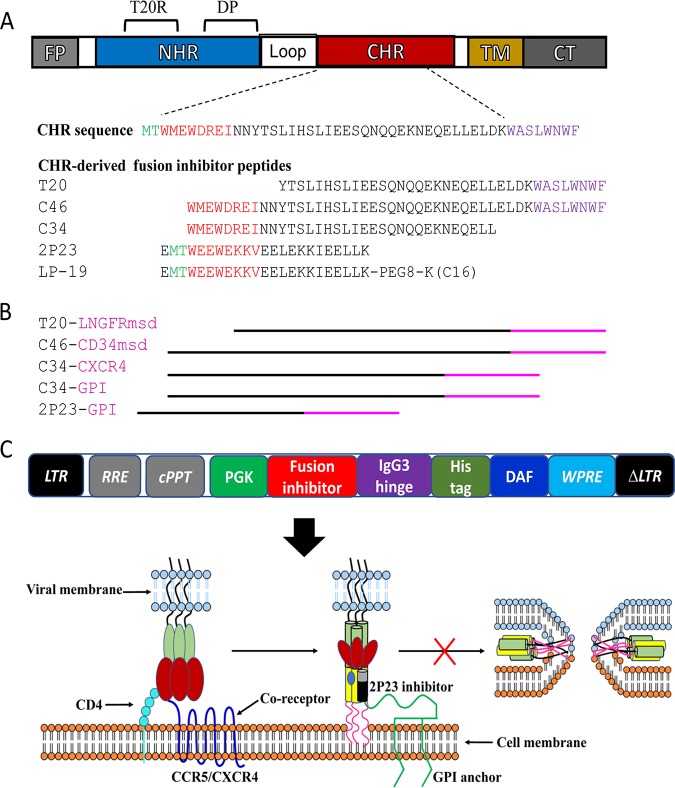
FIG 1.
Diagram of HIV fusion protein gp41 and design strategy for membrane-anchored fusion inhibitors. (A) Functional domains of gp41 and fusion inhibitor peptides. The sequences of the M-T hook structure, pocket-binding domain (PBD), and tryptophan-rich motif (TRM) in the CHR or CHR-derived fusion inhibitor peptides are marked in green, red, and purple, respectively. PEG8 indicates a flexible linker of 8-unit polyethylene glycol; C16 in parentheses indicates palmitic acid. FP, fusion peptide; NHR, N-terminal heptad repeat; CHR, C-terminal heptad repeat; TM, transmembrane domain; CT, cytoplasmic tail; T20RS, T20-resistant site; DP, deep-pocket site. (B) Illustration of membrane-anchored fusion inhibitors. T20 is fused with the membrane-spanning domain (MSD) of low-affinity nerve growth factor receptor (LNGFR), C46 is fused with the MSD of human tCD34, C34 is fused with the coreceptor CXCR4 or attached to glycosylphosphatidylinositol (GPI), and 2P23 is attached to GPI. (C) Lentiviral vector expressing a GPI-anchored fusion inhibitor and its mechanism of action. The encoding sequence of a fusion inhibitor peptide was genetically linked with the sequences encoding the IgG3 hinge region, a His tag, and the GPI attachment signal of DAF. When 2P23 is expressed on the cell surface via a GPI anchor, it binds to the NHR target during the prehairpin state of gp41 and blocks 6-HB formation. LTR, long terminal repeat. RRE, Rev response element; cPPT, central polypurine track; WPRE, woodchuck hepatitits virus posttranscription regulatory element.