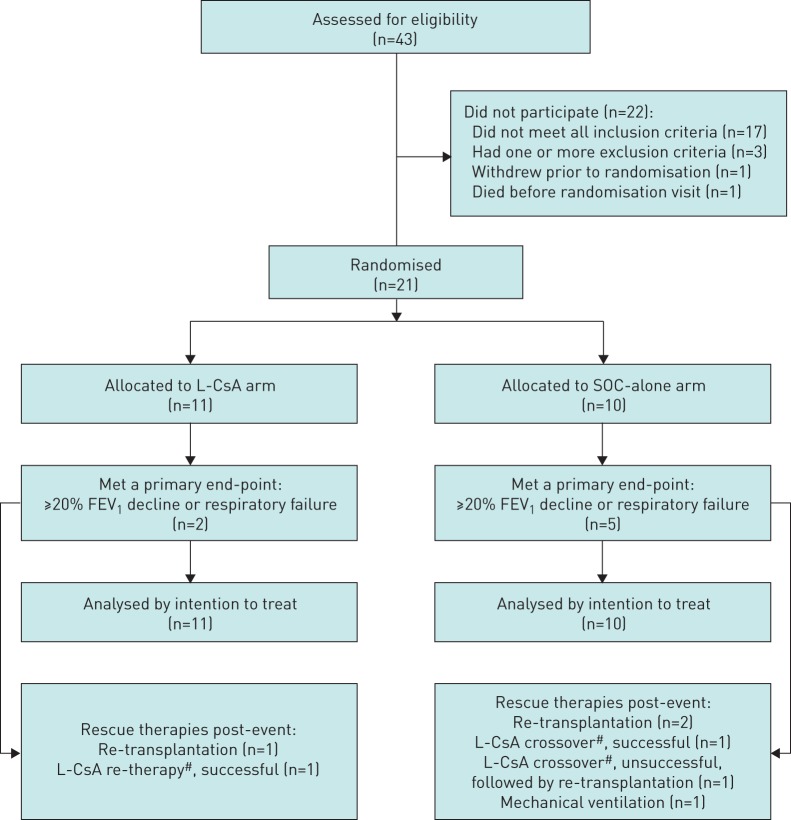
FIGURE 1.
Study enrolment. L-CsA: liposomal cyclosporine; SOC: standard of care; FEV1: forced expiratory volume in 1 s; BOS: bronchiolitis obliterans syndrome. 43 patients were assessed for eligibility for this study. 17 screened patients did not meet BOS grade 1 or 2 criteria and three patients met exclusion criteria. 23 patients met eligibility criteria. One patient died and one patient withdrew prior to randomisation. 21 patients were randomised: 11 patients to the inhaled L-CsA treatment arm given in addition to conventional oral immunosuppression (SOC) and 10 patients to the SOC-alone arm. Patients were followed until an efficacy end-point occurred (a ≥20% FEV1 decline or re-transplantation or death) or until week 48. If the efficacy end-point event occurred before week 48 in the SOC-alone arm, crossover to L-CsA was permitted. If the efficacy end-point occurred in the L-CsA group during the 24-week observation interval only, re-treatment with L-CsA was possible if patients still fulfilled eligibility criteria. One SOC-alone patient developed protracted respiratory failure (>3 weeks duration) due to progressive BOS. #: the mean duration of L-CsA crossover or re-therapy was 156 days (a successful L-CsA “crossover” or “re-therapy” was defined as absence of ≥20% FEV1 decline relative to the time of initiation, according to the end-point definition).