Abstract
Background:
Whole-body vibration (WBV) and mental workload (MWL) are common stressors among drivers who attempt to control numerous variables while driving a car, bus, or train.
Objective:
To examine the individual and combined effects of the WBV and MWL on the autonomic nervous system.
Methods:
ECG of 24 healthy male students was recorded using NeXus-4 while performing two difficulty levels of a computerized dual task and when they were exposing to WBV (intensity 0.5 m/s2; frequency 3–20 Hz). Each condition was examined for 5 min individually and combined. Inter-beat intervals were extracted from ECG records. The time-domain and frequency-domain heart rate variability parameters were then extracted from the inter-beat intervals data.
Results:
A significant (p=0.008) increase was observed in the mean RR interval while the participants were exposed to WBV; there was a significant (p=0.02) reduction in the mean RR interval while the participants were performing the MWL. WBV (p=0.02) and MWL significantly (p<0.001) increased the standard deviation of normal-to-normal intervals with a moderate-to-large effect size. All active periods increased the low-frequency component and low-frequency/high-frequency ratio. However, only the WBV significantly increased the highfrequency component. A significant (p=0.01) interaction was observed between the WBV and MWL on low-frequency component and low-frequency/high-frequency ratio.
Conclusion:
Exposure to WBV and MWL can dysregulate the autonomic nervous system. WBV stimulates both sympathetic and parasympathetic nervous system; MWL largely affects sympathetic nervous system. Both variables imbalance the sympatho-vagal control as well.
Keywords: Autonomic nervous system, Vibration, Mental processes, Workload, Heart rate
TAKE-HOME MESSAGE
Whole body vibration (WBV) and mental workload (MWL) are common stressors among drivers.
All active periods increased the low frequency component and the low frequency/high frequency ratio.
WBV significantly increased the high frequency component.
A interaction effect was observed between the WBV and MWL on the low frequency component and the low frequency/ high frequency ratio.
Concomitant exposure to WBV and MWL could dysregulate the autonomic nervous system.
Introduction
How drivers respond to the environmental factors, is a key element in safety and ergonomics. They are under various stressors including whole body vibration (WBV) in the range of 1–20 Hz,1,2 mental workload (MWL) and ergonomics factors.3,4 Some evidence suggests that the mental processing and physical stressors might dysregulate the autonomic nervous system.5,6 These, in turn, might associate with heart diseases, obesity, diabetes, metabolic disorders, as well as common features of the biology of acute and chronic stress.7
The imbalance in the autonomic nervous system might be revealed by the examination of oscillations in the interval between heartbeats (R-R intervals, RRI), the so-called “heart rate variability.”8 Researchers have used heart rate variability, with an emphasis on the time domain variables including the mean RR, the standard deviation (SD) of normal-to-normal RR intervals, and the root mean square (RMS) of the successive differences, and the frequency domain variables including low-frequency (LF, sympathetic response) component, high-frequency (HF, parasympathetic response) component, and LF/HF ratio (sympatho-vagal balance) to ascertain whether different stressors might affect the autonomic nervous system.9
Ryu and Myung showed that body response to a dual task of tracking and mental arithmetic includes sympatric innervation and decreased mean RR interval.10. Fallahi, et al, found a significant positive correlation between the traffic congestion and heart rate, RMS of the successive differences and the standard deviation of normal-to-normal intervals as well as LF/HF ratio.11 Overall, the level of laboratory mental stressors such as mental arithmetic, reaction time tasks, or Stroop task,10,12,13 and real-life mental stressors such as driving or traffic monitoring,11,14 directly correlates to the level of LF component and LF/HF ratio and inversely correlates to the HF component.15
Available studies on the responses of the autonomic nervous system to occupational WBV have generally indicated degrees of dysfunction and autonomic imbalance.16-19 Harstela and Piirainen noted that exposure to WBV may have a significant effect on decreasing heart rate variability; this can be more significant when the subject is under mental stress.16 Jiao, et al, reported that WBV might significantly activate the sympathetic and suppress the parasympathetic nervous system leading to increased LF/HF ratio.18 In another study, Zhang, et al, suggested that concurrent performing of a driving task and exposure to WBV increases the LF/HF ratio and decreases the RMS of the successive differences of the heart rate. The combined effect of driving and WBV on LF/HF ratio is more pronounced than that of the driving without WBV.20
There are just a few studies on the relationship between the human autonomic nervous system responses and combined effects of exposure to WBV and MWL; only a few studied the issue using advanced analyses of heart rate variability and the individual. Furthermore, recent studies have not reported the effect size of these independent variables on the autonomic nervous system. The current study was thus conducted to find how MWL individually or in combination with WBV might affect the autonomic nervous system activity.
Materials and Methods
Participants
Twenty-four healthy right-handed male university students with a mean age of 24 (SD 4) years and BMI of 23 (1) kg/m2 voluntarily participated in this study. Participants had normal or corrected-to-normal vision. Those with history of any diseases or continuous alcohol consumption, smoking, or drugs abuse were excluded from the study. The participants were asked not to drink coffee three hours before the experiment. The protocol was approved by Shiraz University of Medical Sciences Ethics Committee. An informed written consent was obtained from each participant.
Vibration Simulator and Monitoring
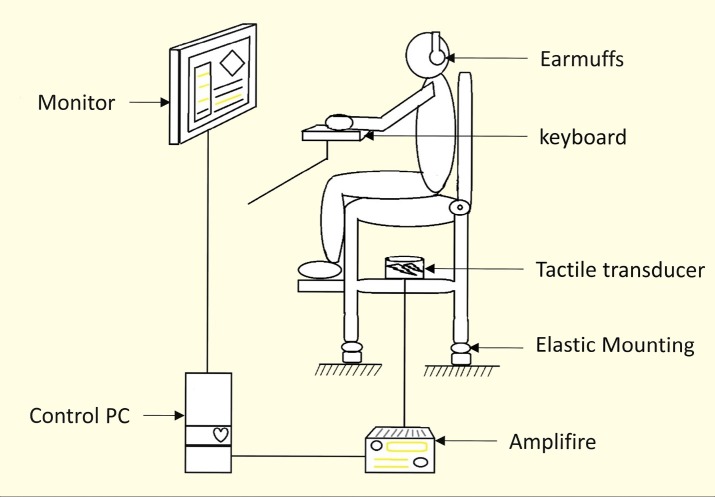
A customized vibration simulator with the ability to create 3D vibration at different frequencies was utilized in this study. The vibration simulator included an adjustable car seat (Peugeot 405 driver seat, IK Co, Tehran, Iran) attached to a metal frame structure. A tactile transducer (ButtKicker LFE transducer, The Guitammer Co, Westerville, OH, USA) was placed on the center of a metal structure, 25 cm above the ground. A signal was generated using NI LabVIEW 2012 (National Instruments, Austin, TX, USA) and amplified using a 1000 W amplifier (ButtKicker BKA1000-N Power Amplifier, The Guitammer Co, Westerville, OH, USA). The signal was then transmitted to the transducer. This device could produce a three axial sine or random waves at different frequencies and intensities. The applied unweighted vibration was set to sine waves with a frequency of 3–20 Hz and an intensity of 0.5 m/s2. The generated vibration on the supporting seat surface was monitored real time using an SVAN 958 vibration analyzer with an SV 39A whole-body seat accelerometer (SVANTEK Sp. z oo, Warsaw, Poland) to ensure the calibration of the vibration (Fig 1).
Figure 1.
An illustration of vibration simulator when a subject is using the experimental setup.
Computerized Dual Task and Presentation
Using C#, a software program was developed to determine the compensatory tracking task and the choice reaction time task. To complete the tasks, participants were guided to try positioning a horizontal bar that continuously left its target reference point by pressing the up and down keys on a keyboard. Cursor velocities of 80 and 160 pixel/s were used to obtain the low and high levels of MWL, respectively. In another task, the choice reaction time task, a blue number (2, 3, 4, or 5) was randomly shown up on a yellow background, on the left side of a monitor. The participants were instructed to press ‘A’ for numbers ‘2’ or ‘3’ and ‘S’ for ‘4’ or ‘5,’ once they appeared on the screen.
Electrocardiogram Recording and Analysis
A NeXus-4 (Mind Media BV, the Netherlands) was used to record ECG for each participant. The heart signals were collected at sampling frequency of 1024 Hz from three Ag-AgCl electrodes placed at the distal part of the sternum and at the sixth intercostal space in the left axilla. The ECG signals were collected by the BioTrace+® software (Media BV, Roermond-Herten, the Netherlands) and analyzed by the Kubios HRV ver 3.0.0 (Biomedical Signal Analysis Group, Department of Applied Physics and University of Kuopio, Finland)21 based on the time domain and frequency domain analyses (autoregressive model order 16). Time domain variables were including the mean RR (ms), SD of normal-to-normal intervals (ms), and RMS of the successive differences (ms). Frequency domain variables included LF (normalized unit [nu]), HF (nu) and LF/HF ratio. A threshold of 5% was applied to filter the signals.
Procedure
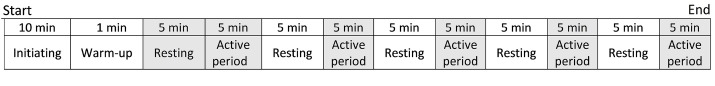
The participants were invited to an environmentally controlled lab with a noise level of 59 (SD 3) dB-A, temperature of 22 (2) °C, and lighting level of 510 (20) lux between 8:00 and 13:00. They completed a trial while seated on the simulator seat. At the first 10 minutes of the trial, the three electrodes were attached to the participants and NeXus-4 was prepared to record ECG. The participants were instructed to complete the computerized dual task following 1-minute warm-up. Then, they rested for five minutes. In the next step, five active periods including exposure to WBV, handling low-level MWL, handling high-level MWL, concurrent exposure to WBV and low-level MWL (WBV.LMWL), and concurrent exposure to WBV and high-level MWL (WBV.HMWL) were presented to the participants for five minutes. A 5-minute resting period was considered between every two active periods. Generally, the trial took an hour for each participant. ECG was recorded during the first resting period (5 minutes) and during active periods (totally 25 minutes). The order of tasks (active periods) assigned to each participant was random (Fig 2).
Figure 2.
Schematic illustration of the trial periods and trend. Active periods include exposure to whole body vibration (WBV), handling low-level mental workload (MWL), handling high-level MWL, concurrent exposure to WBV and handling low-level MWL, and concurrent exposure to WBV and handling high-level MWL. ECG was recorded during the shaded periods.
Statistical Analysis
The mean and interaction effects of the independent variables on heart rate variability were assessed with repeated-measure ANOVA followed by the least significant difference as the post hoc test to assess the within-subject relationship. Additionally, the Cohen's d statistic was applied to calculate the effect size.22 SPSS® for Windows® ver 20 (SPSS Inc, Chicago, IL, USA) was utilized for statistical analysis. GraphPad Prism ver 7 (GraphPad Software, San Diego, CA, USA) was used for graphical illustrations.
Results
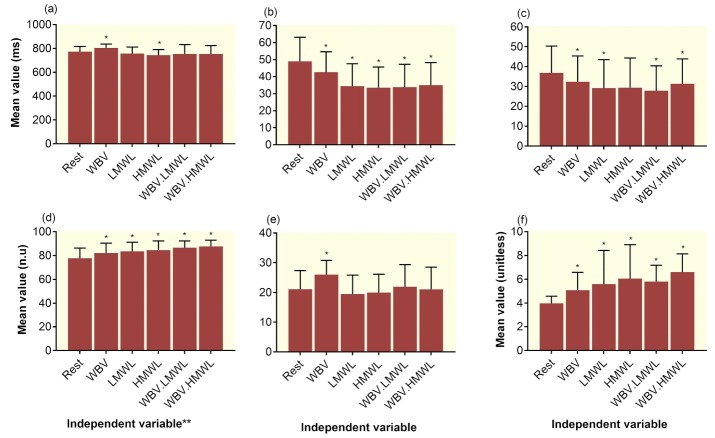
The mean RR interval was significantly (p<0.008) increased by WBV (Fig 3a). However, compared with the resting period, it was significantly (p=0.02) decreased when the participants handled the HMWL. A significant decrease in the mean value of the SD of normal-to-normal intervals (pWBV=0.02, pLMWL<0.001, pHMWL<0.001, pWBV.LMWL=0.001, and pWBV.HMWL<0.001) (Fig 3b) and RMS of the successive differences (pWBV=0.04, pLMWL=0.02, pWBV.LMWL=0.03, and pWBV.HMWL=0.05) were observed when the results were compared between the resting and active periods (Fig 3c).
Figure 3.
Comparison of the time-domain variables including (a) Mean RR (ms), (b) SD of normal-to-normal intervals (ms), and (c) root mean square of the successive differences (ms); and frequency-domain variables including (d) low-frequency (LF) (n.u), (e) high-frequency (HF) (n.u), and (f) LF/HF ratio between the resting and active periods. Error bars represent the SD. *Significant difference (p<0.05) between resting and active periods **WBV: Whole-body vibration, LMWL: Low mental workload, HMWL: High mental workload, WBV.LMWL: Combined WBV and LMWL, and WBV.HMWL: Combined WBV and HMWL
Two frequency domain variables, LF component and LF/HF ratio, were significantly increased when the participants involved in active tasks (pWBV=0.03, 0.04; pLMWL=0.004, 0.001; pHMWL=0.008, <0.001; pWBV.LMWL<0.001, 0.002; pWBV.HMWL<0.001, <0.001, respectively) (Figs 3d and 3f). The HF component, however, was increased significantly (p=0.05) only when participants exposed to the WBV (Fig 3e).
A significant incremental effect of WBV on the mean RR and SD of normal-to-normal intervals was also observed in time and frequency domain heart rate variability during active periods (Tables 1 and 2). Compared with WBV per se, WBV.MWLs significantly induced a higher level of LF component (Table 2). WBV induced a significantly higher level of HF component compared with LMWL, HMWL and WBV.HMWL. Finally, we found that exposure to HMWL and WBV.HMWL could induce a significant higher LF/HF ratio compared with both WBV and LMWL.
| Table 1: Comparison of time-domain heart rate variability parameters between the active periods | ||||||||
| Active period* | Mean RR | SDNN † | RMSSD ‡ | |||||
| Mean difference | p value | Mean difference | p value | Mean difference | p value | |||
| WBV vs LMWL | 47.07 | 0.01 | 8.60 | 0.002 | 3.16 | 0.39 | ||
| WBV vs HMWL | 59.40 | 0.001 | 9.20 | 0.02 | 2.96 | 0.52 | ||
| WBV vs WBV.LMWL | 50.20 | 0.01 | 8.73 | 0.009 | 4.49 | 0.29 | ||
| WBV vs WBV.HMWL | 50.07 | 0.01 | 6.87 | 0.01 | 0.96 | 0.77 | ||
| LMWL vs HMWL | 12.33 | 0.41 | 0.60 | 0.85 | -0.20 | 0.95 | ||
| LMWL vs WBV.LMWL | 3.13 | 0.82 | 0.13 | 0.93 | 1.33 | 0.66 | ||
| LMWL vs WBV.HMWL | 3.00 | 0.85 | -1.73 | 0.50 | -2.20 | 0.41 | ||
| HMWL vs WVB.LMWL | -9.20 | 0.56 | -0.47 | 0.90 | 1.53 | 0.44 | ||
| HMWL vs WBV.HMWL | -9.33 | 0.54 | -2.33 | 0.36 | -2.00 | 0.38 | ||
| WBV.LMWL vs WBV.HMWL | -0.13 | 0.99 | -1.87 | 0.45 | -3.53 | 0.12 | ||
| *WBV: Whole-body vibration, LMWL: Low mental workload, HMWL: High mental workload †SDNN: SD of normal-to-normal intervals, ‡RMSSD: root mean square of the successive differences | ||||||||
| Table 2: Comparison of frequency-domain heart rate variability parameters between the active individual and combined periods | ||||||||
| Active period* | Low frequency (LF) | High frequency (HF) | LF/HF ratio | |||||
| Mean difference | p value | Mean difference | p value | Mean difference | p value | |||
| WBV vs LMWL | -0.64 | 0.79 | 6.56 | 0.005 | -0.31 | 0.49 | ||
| WBV vs HMWL | -2.38 | 0.49 | 5.98 | 0.005 | -1.43 | 0.009 | ||
| WBV vs WBV.LMWL | -4.42 | 0.06 | 4.05 | 0.10 | -0.73 | 0.27 | ||
| WBV vs WBV.HMWL | -5.51 | 0.04 | 4.95 | 0.02 | -1.52 | 0.03 | ||
| LMWL vs HMWL | -1.74 | 0.45 | -0.58 | 0.79 | -1.12 | 0.02 | ||
| LMWL vs WBV.LMWL | -3.78 | 0.10 | -2.51 | 0.27 | -0.41 | 0.50 | ||
| LMWL vs WBV.HMWL | -4.87 | 0.06 | -1.61 | 0.42 | -1.20 | 0.04 | ||
| HMWL vs WVB.LMWL | -2.03 | 0.41 | -1.93 | 0.19 | 0.71 | 0.16 | ||
| HMWL vs WBV.HMWL | -3.12 | 0.27 | -1.03 | 0.70 | -0.08 | 0.86 | ||
| WBV.LMWL vs WBV.HMWL | -1.09 | 0.51 | 0.89 | 0.71 | -0.79 | 0.86 | ||
| *WBV: Whole-body vibration, LMWL: Low mental workload, HMWL: High mental workload | ||||||||
Figure 4 illustrates the estimated effect sizes of the time domain (mean RR, SD of normal-to-normal intervals, and RMS of the successive differences) and frequency domain (LF, HF, and LF/HF ratio) heart rate variability parameters when a comparison was made between resting and active periods. Amongst the time domain variables, the effect size was moderate or less for the mean RR (Fig 4a) and RMS of the successive differences (Fig 4c). A large effect size was observed for the SD of normal-to-normal intervals for all active periods except for WBV (Fig 4b). The magnitude was small for the LF component and moderate for the WBV and MWL. Nonetheless, the combined effects of these independent variables resulted in a large effect size (Fig 4c). The effect size of the HF component was small for WBV; that of other variables was negligible (Fig 4e). Finally, although the LF/HF ratio had a small change with WBV, it had a large change with MWLs and combined variables. The highest observed effect size of 1.79 was related to the LF/HF ratio when a comparison was made between the resting and HMWL periods.
Figure 4.
An illustration of the effect size of the time-domain variables including (a) Mean RR (ms), (b) SD of normal-to-normal intervals (ms), and (c) root mean square of the successive differences (ms); and frequency-domain variables including (d) low-frequency (LF) (n.u), (e) high-frequency (HF) (n.u), and (f) LF/HF ratio *WBV: Whole-body vibration, LMWL: Low mental workload, HMWL: High mental workload, WBV.LMWL: Combined WBV and LMWL, and WBV.HMWL: Combined WBV and HMWL
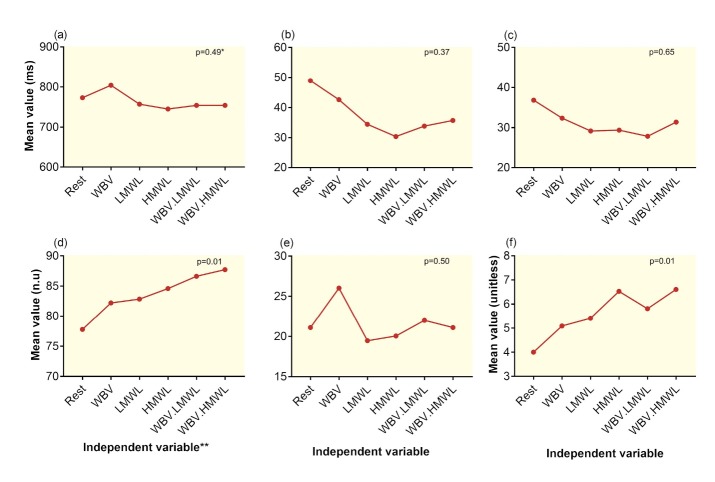
Figure 5 shows the trend of changes in the time and frequency domain heart rate variability parameters and independent variable interactions. Generally, all active periods decreased the means of the time domain variables, except for WBV that increased the mean RR. No significant interaction was observed between the WBV and MWL in terms of time domain variables. There was a significant (p=0.01) incremental interaction effect between the WBV and MWLs; the combined effect of these variables showed a larger effect on the LF component and LF/HF ratio compared with the effect of each one per se (Figs 5d and 4e). MWL had a slight non-significant antagonistic effect on the incremental effect of WBV on HF (Fig 5e).
Figure 5.
Trend of changes and interaction effects of independent variables on the time-domain variables including (a) Mean RR, (b) SD of normal-to-normal intervals, (c) root mean square of the successive differences; and frequency-domain variables including (d) low frequency (LF) (n.u), (e) high frequency (HF) (n.u), and (f) LF/HF ratio across the rest and active periods *p value for the interaction between WBV and MWL **WBV: Whole-body vibration, LMWL: Low mental workload, HMWL: High mental workload, WBV.LMWL: Combined WBV and LMWL, and WBV.HMWL: Combined WBV and HMWL
Discussion
We found that WBV could significantly increase the mean RR interval; HMWL had an opposite effect. All active periods significantly decreased the SD of normal-to-normal interval, compared with the resting period. WBV significantly increased the mean LF and HF components; MWL increased the mean LF component; all active periods significantly increased the mean LF/HF ratio, compared with the resting period. Trend and interaction effect analysis indicated a significant positive interaction between the WBV and MWLs—while the combination of these two variables had a larger effect on the LF component and LF/HF ratio than the effect of each variable alone.
A large body of evidence suggests that MWL can directly reduce the mean RR interval, SD of normal-to-normal intervals, and RMS of the successive differences.11,15,23 Several studies report that the mean LF component and LF/HF ratio increase when one takes on a mental task, with slight reduction in the HF component.11,15,24 Visnovcova, et al, report that a high level of mental stress can significantly reduce the mean RR and SD of normal-to-normal intervals in a group of healthy men. They also report increased level of LF component and a confirmed vagal withdrawal (lower HF).25 Similar findings have been reported by Scheer, et al,26 and Okawa, et al, that taking on a mental task stimulated the sympathetic nervous system. Jiao, et al, show that after a simulated driving sympathetic activity of the participants is enhanced while parasympathetic activities slightly decrease.27 The findings of the current study were in line with recent reports on the association between the autonomic nervous system response and taking on mental tasks.
Generally, there is a paucity of research designed to investigate the relationship of vibration exposure (frequency range of up to 20 Hz) and autonomic nervous system response so that most of the published articles have examined the heart rate variability parameters when participants were completing a driving task on a vibrating simulator; they report increased sympathetic nervous system activity along with increased mean LF/HF ratio.18,20 Zhang, et al, report that taking on a driving task under vibrational condition (3–7 Hz) increases the LF/HF ratio and decreases the RMS of the successive differences. Additionally, they suggest that the incremental effect of combined WBV and mental stress on LF/HF ratio is more pronounced than that for driving without being exposed to WBV.20 The study of Jiao, et al, suggests that completing a driving task under WBV condition (1.8 and 6 Hz) significantly increases the LF component and LF/HF ratio and decreases the HF component. Moreover, they show that the autonomic nervous system response (increasing LF and LF/HF ratio) to the combined effect of mental stress and WBV is more pronounced than that for driving without exposure to vibration.18
The current study findings on sympathetic activity, sympatho-vagal imbalance and the potential effect of simultaneous exposure to WBV and a mental stressor on heart rate variability components were consistent with the findings of recent studies. This study, however, suggested an inconsistent finding about the parasympathetic activity, ie, the increased level of parasympathetic activity when participants were exposed to WBV. It has been claimed that the autonomic nervous system response to WBV strongly depends on the frequency characteristics of the vibration rather than its direction or amplitude.18,28 This inconsistency could be attributed to the difference in the studied frequencies.
Some limitations to this pilot study need to be acknowledged. The sample size was relatively small; women were not included in the present study. Furthermore, we could not test the effect of every single vibration frequency on participants. The current study only examined the heart rate variability; other objective tests such as galvanic skin response could be very helpful to better answer the research question.
In conclusion, the current study suggested that exposure to WBV and a mental stressor could significantly increase the sympatric activity and imbalance sympatho-vagal control. Human exposure to WBV at the frequency range of 3–20 Hz might significantly stimulate parasympathetic activity. Concurrent exposure to WBV and a mental stressor could have synergistic or antagonistic effects on the autonomic nervous system response.
Acknowledgments
The current paper was extracted from the PhD thesis of Hamed Jalilian. We would like to thank the studied students for participating in this study and Dr. Alireza Choobineh, Research Center for Health Sciences, Institute of Health, Shiraz University of Medical Sciences, Shiraz, Iran, for his kind support.
Conflicts of Interest:
None declared.
Financial Support:
The study was supported by Shiraz University of Medical Sciences (grant No. 11607).
Cite this article as: Jalilian H, Zamanian Z, Gorjizadeh O, et al . Autonomic nervous system responses to wholebody vibration and mental workload: A pilot study. Int J Occup Environ Med 2019;10:174-184. doi: 10.15171/ ijoem.2019.1688
References
- 1. Meram A, Shahriari M. Evaluation of Whole-Body Vibration in Automobile on Routine Travel—A Case Study.In: Arezes PM, Baptista JS, Barroso MP, et al, eds. Occupational and Environmental Safety and Health. Springer, 2019:191-9.
- 2.Emkani M, Hashemi Nejad N, Jalilian H. et al. Exposure to whole body vibration in heavy mine vehicle drivers and its association with upper limbs musculoskeletal disorders. Journal of Occupational Health and Epidemiology. 2016;5:226–34. [Google Scholar]
- 3.Hassanzadeh-Rangi N, Khosravi Y, Farshad AA, Jalilian H. [Workloadin Train Driving Job: AffectingFactors and Improvement Recommendations] Journal of Ergonomics. 2016;5:60–72. [in Persian]. [Google Scholar]
- 4.Hassanzadeh-Rangi N, Khosravi Y, Farshad AA, Jalilian H. [Assessment and analysis of physical workload in metro driving job and recommendation for improvement] Journal of Health and Safety at Work. 2017;7:33–44. [in Persian]. [Google Scholar]
- 5.Delliaux S, Delaforge A, Deharo JC, Chaumet G. Mental Workload Alters Heart Rate Variability, Lowering Non-linear Dynamics. Frontiers in Physiology. 2019;10 doi: 10.3389/fphys.2019.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh N, Aggarwal Y, Sinha RK. Heart rate variability analysis under varied task difficulties in mental arithmetic performance. Health and Technology. 2019;9:343–53. [Google Scholar]
- 7.Wulsin L, Herman J, Thayer JF. Stress, autonomic imbalance, and the prediction of metabolic risk: A model and a proposal for research. Neurosci Biobehav Rev. 2018;86:12–20. doi: 10.1016/j.neubiorev.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 8. Vigo DE, Siri LN, Cardinali DP. Heart Rate Variability: A Tool to Explore Autonomic Nervous System Activity in Health and Disease. In: Gargiulo PÁ, Mesones Arroyo HL, eds. Psychiatry and Neuroscience Update : From Translational Research to a Humanistic Approach - Volume III. Cham, Springer International Publishing, 2019:113-26.
- 9.Electrophysiology Task Force of the European Society of Cardiology the North American Society of Pacing. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 10.Ryu K, Myung R. Evaluation of mental workload with a combined measure based on physiological indices during a dual task of tracking and mental arithmetic. Int J Ind Ergon. 2005;35:991–1009. [Google Scholar]
- 11.Fallahi M, Motamedzade M, Heidarimoghadam R. et al. Effects of mental workload on physiological and subjective responses during traffic density monitoring: A field study. Appl Ergon. 2016;52:95–103. doi: 10.1016/j.apergo.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Kim HG, Cheon EJ, Bai DS. et al. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018;15:235–45. doi: 10.30773/pi.2017.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kofman O, Meiran N, Greenberg E. et al. Enhanced performance on executive functions associated with examination stress: Evidence from task-switching and Stroop paradigms. Cognition and Emotion. 2006;20:577–95. [Google Scholar]
- 14.Thwe PP, Yamamoto T, Sato H, Morikawa T. Analysis of Driving Stress on Various Roadway Conditions in Myanmar by using Heart Rate Variability. Asian Transport Studies. 2017;4:663–79. [Google Scholar]
- 15.Castaldo R, Melillo P, Bracale U. et al. Acute mental stress assessment via short term HRV analysis in healthy adults: A systematic review with meta-analysis. Biomedical Signal Processing and Control. 2015;18:370–7. [Google Scholar]
- 16.Harstela P, Piirainen K. Effect of whole-body vibration and driving a forest machine simulator on some physiological variables of the operator. Silva Fennica. 1985;19:197–202. [Google Scholar]
- 17.al-Nashash H, Qassem W, Zabin A, Othman M. ECG response of the human body subjected to vibrations. J Med Eng Technol. 1996;20:2–10. doi: 10.3109/03091909609032524. [DOI] [PubMed] [Google Scholar]
- 18.Jiao K, Li Z, Chen M. et al. Effect of different vibration frequencies on heart rate variability and driving fatigue in healthy drivers. Int Arch Occup Environ Health. 2004;77:205–12. doi: 10.1007/s00420-003-0493-y. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Shin K, Lee S. et al. Ride comfort analysis with physiological parameters for an e-health train. Telemed J E Health. 2009;15:1010–21. doi: 10.1089/tmj.2009.0040. [DOI] [PubMed] [Google Scholar]
- 20.Zhang N, Fard M, Bhuiyan MHU. et al. The effects of physical vibration on heart rate variability as a measure of drowsiness. Ergonomics. 2018;61:1259–72. doi: 10.1080/00140139.2018.1482373. [DOI] [PubMed] [Google Scholar]
- 21.Tarvainen MP, Niskanen JP, Lipponen JA. et al. Kubios HRV–heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113:210–20. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luque-Casado A, Perales JC, Cárdenas D, Sanabria D. Heart rate variability and cognitive processing: The autonomic response to task demands. Biol Psychol. 2016;113:83–90. doi: 10.1016/j.biopsycho.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Hsu BW, Wang MJ, Chen CY, Chen F. Effective indices for monitoring mental workload while performing multiple tasks. Percept Mot Skills. 2015;121:94–117. doi: 10.2466/22.PMS.121c12x5. [DOI] [PubMed] [Google Scholar]
- 25.Visnovcova Z, Mestanik M, Javorka M. et al. Complexity and time asymmetry of heart rate variability are altered in acute mental stress. Physiol Meas. 2014;35:1319–34. doi: 10.1088/0967-3334/35/7/1319. [DOI] [PubMed] [Google Scholar]
- 26.Scheer FAJL, Chellappa SL, Hu K, Shea SA. Impact of mental stress, the circadian system and their interaction on human cardiovascular function. Psychoneuroendocrinology. 2019;103:125–9. doi: 10.1016/j.psyneuen.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao K, Li ZY, Chen M, Wang CT. Power spectral analysis of heart rate variability of driver fatigue. Journal of Donghua University. 2005;22:11–5. [Google Scholar]
- 28.Kimura H, Endo M, Koseki M, Inou N. Sleep-Inducing Factors in Mechanical Environments. Journal of Environment and Engineering. 2010;5:275–86. [Google Scholar]