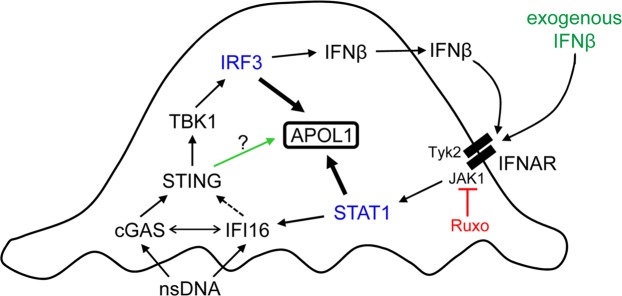
Figure 9.
Our proposed model of nsDNA-induced APOL1 expression through engagement of the cGAS/IFI16-STING pathway in human immortalized AB8/13 podocytes. Binding of cytosolic nsDNA by cGAS and IFI16 activates STING, which subsequently activates TBK1. Activated TBK1 phosphorylates IRF3, which promotes transcription of APOL1 and IFNβ. IFNβ released from the cells (or exogenous IFNβ) binds to IFNAR. IFNAR-associated JAK1 and Tyk2 kinases then phosphorylate STAT1, which promotes transcription of APOL1 and IFI16. A putative IFI16-mediated activation of STING is indicated by a dashed arrow. A potential cooperation between cGAS and IFI16 is indicated by a double-headed arrow. Deficient STING phosphorylation observed in cGAS- or IFI16-knockdown cells (Fig. 6) suggests that nsDNA-induced APOL1 expression may be mediated by a phospho-STING-independent pathway, marked by a green arrow. A dual JAK1/JAK2 inhibitor Ruxolitinib (Ruxo) suppresses STAT1 activation and thereby inhibits IFI16 expression and STAT1-mediated APOL1 expression. Since IFNAR-mediated signaling involves JAK1 but not JAK2, our model only depicts the inhibition of JAK1 by Ruxo. Ruxo does not affect IRF3-mediated APOL1 expression.