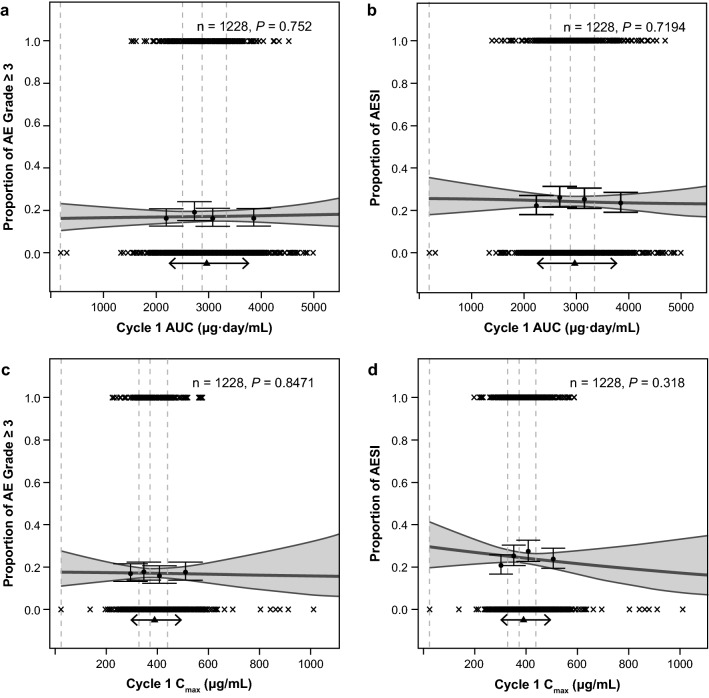
Fig. 3.
Pooled exposure–response analyses of safety in patients with locally advanced or metastatic NSCLC or UC. Indicated AE frequencies ([a, c] grade ≥ 3 AEs; [b, d] AESIs) are plotted vs (a, b) AUC or (c, d) Cmax at cycle 1. For legibility, 2 extreme AUC values (> 15,000 μg.day/mL) and 2 extreme Cmax values (> 1500 μg/mL) are not displayed on the plots. Wald P values from logistic regression of AE incidence vs exposure are displayed. Gray solid lines and shaded areas represent the logistic regression slope model and 95% PI. Filled circles and error bars represent AE proportion in exposure quartiles and 95% CI; vertical lines are the limits of the exposure quartiles. Cross markings (×) represent AE events (0: no, 1: yes). Triangle and two-headed arrows represent the mean exposure and exposure interval between the 10th and 90th percentiles, respectively, for patients receiving atezolizumab 1200 mg. Cycle 1 AUC corresponds to the AUC during the first 3 weeks after treatment start and with PK parameters estimated based on cycle 1 data only. AE adverse event, AESI adverse event of special interest, AUC area under the concentration–time curve, Cmax maximum serum atezolizumab concentration, n number of patients, NSCLC non-small cell lung cancer, PI prediction interval, PK pharmacokinetics, UC urothelial carcinoma