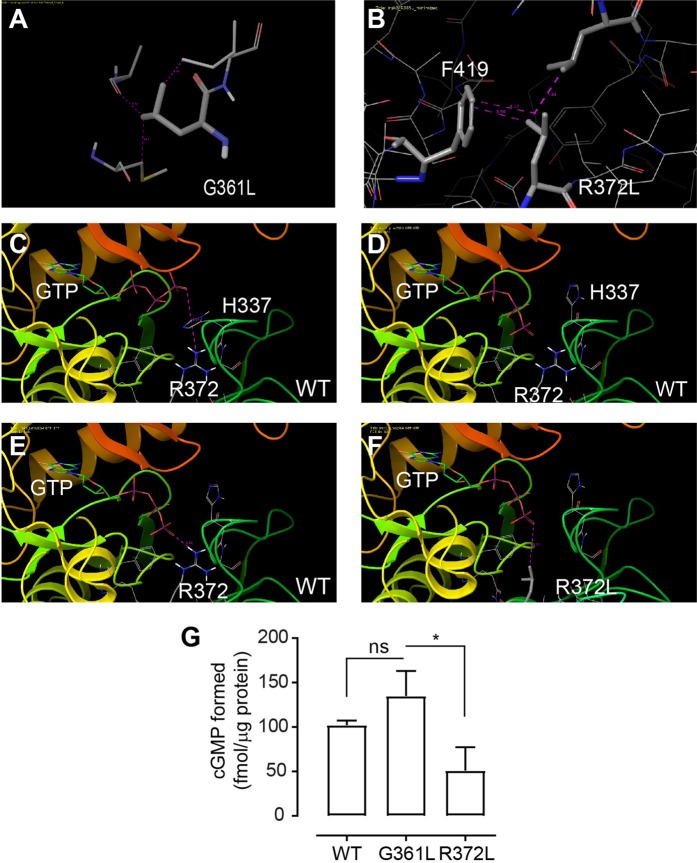
Figure 2.
A mutation in the guanylate cyclase centre can suppress cGMP production by IRAK3. (A,B) Close up models of mutated versions of the human IRAK3 protein at the guanylate cyclase centre showing the G361L (A) and the R372L (B) mutations in the absence of GTP. (C–E) Close up of GTP docking interactions at the guanylate cyclase centre in wildtype (WT) human IRAK3. (C) Interaction between the γ phosphate of GTP and R372 in the wildtype protein when H337 interferes. (D) Interaction between the γ phosphate of GTP and R372 in the wildtype protein when H337 is moved. (E) Interaction between the γ phosphate of GTP and R372 in the wildtype protein at approximately 4 Å. (F) Close up of ribbon model of GTP docked into the guanylate cyclase centre of IRAK3 R372L mutant showing a lack of interaction with the L372 and the γ phosphate of GTP (residues separated by about 6 Å). (G) IRAK3 protein was expressed as either wildtype IRAK3, IRAK3 mutant G361L or IRAK3 mutant R372L. Mutant G361L produced comparable amounts of cGMP to IRAK3 wildtype. Mutant R372L showed significantly decreased cGMP production when compared to G361L mutant (mean ± sem, n = 3–5, *P = 0.0479, one-way ANOVA followed by Tukey’s multiple comparison test).