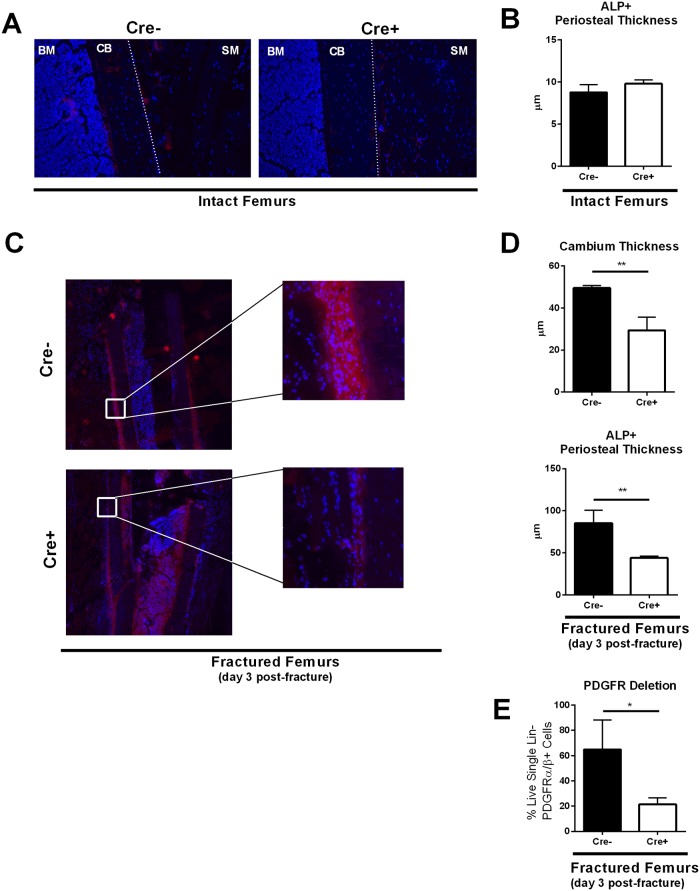
Fig 4. αSMA-Cre-mediated removal of PDGFRβ in periosteal progenitors after bone injury dramatically reduces the periosteal response to fracture seen in gCblYF mice.
(A) Representative images of the periosteal thickness and ALP activity in intact bones. 10x magnification. Blue = DAPI, red = ALP activity stain. BM = bone marrow, CB = cortical bone, dotted lined = periosteum, SM = skeletal muscle. N = 3–4 biological replicates per group. (B) Quantification of ALP activity stain thickness (μm) in intact bones. Tamoxifen (75μg/g body weight) for conditional deletion of PDGFRβ was administered at days 0 and 1 prior to sacrifice. N = 3–4 biological replicates per group. (C) Fractured femurs from Cre- and Cre+ mice at 10x magnification (Table 1). Representative images of the periosteal thickness and ALP activity 1500μm from the fracture site at day 3 post-fracture. Blue = DAPI, red = ALP activity stain. CB = cortical bone, SM = skeletal muscle. Tamoxifen (7.5μL/gram) for conditional deletion of PDGFRβ was administered at days 0 and 1 post-fracture. N = 3–4 biological replicates per group. (D) Quantification of cambium layer thickness and ALP activity stain thickness (μ). Each value represents the average of four measurements at different sites 1500μm away from the fracture site in one sample. N = 3 biological replicates per group. **p<0.005 (E) To confirm PDGFR deletion in our mouse model, we isolated periosteum from the fracture site day three post-fracture from both experimental (Cre+) and control (Cre-) groups. Cells were digested into single cell suspension and stained for flow cytometry analyses. We analyzed the levels of PDGFRα and PDGFRβ in Lin- (CD45- TER119- CD31-) cells to validate our inducible mouse model. N = 3 *p<0.05.