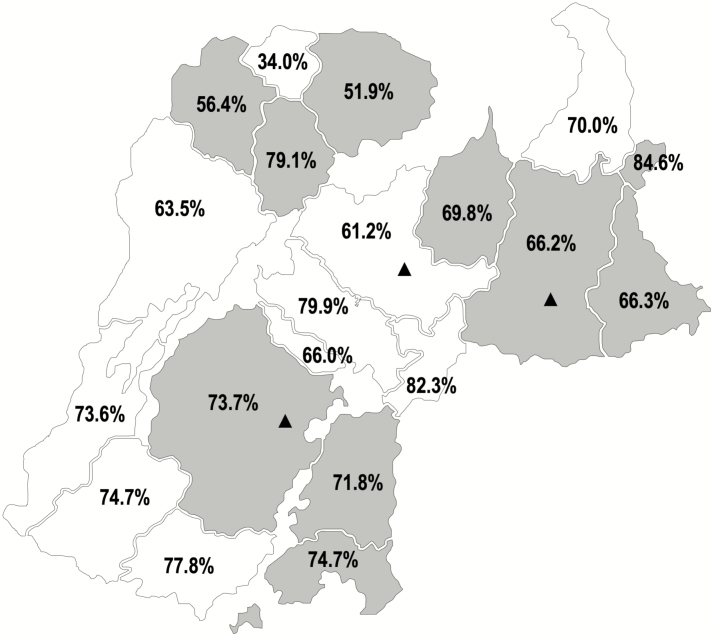
Figure 1.
Geographic distribution of villages randomized to IIV3 and IPV campaigns and achieved village-level vaccination coverage among age-eligible children for the campaigns during the trial, from the Niakhar Demographic Surveillance System. The IIV3 campaigns were conducted in the 10 villages that are shaded gray and the IPV campaigns were conducted in the 10 villages that are shaded white. Coverage is shown as the percent of age-eligible children—6 months through 10 years of age—receiving at least 1 dose. Enhanced, passive surveillance was conducted in the 3 health posts marked with triangles. Abbreviations: IIV3, trivalent inactivated influenza vaccine; IPV, inactivated polio vaccine.