Figure 7.
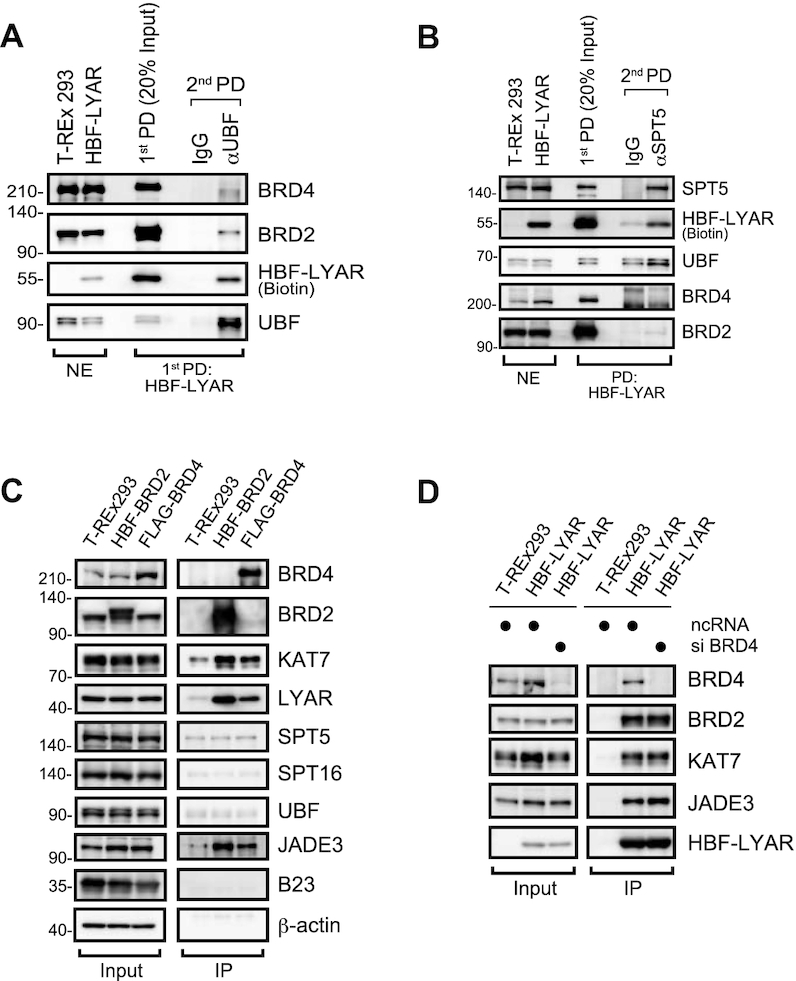
BRD2, BRD4, and SPT5 bind LYAR independently (A, B) Reverse pulldown (PD) of UBF (A) or SPT5 (B) from HBF-LYAR complexes, assessed with immunoblotting. HBF-LYAR-TO cells were treated with Dox for 24 h, and then nuclear extracts (NE) were subjected to first pulldown (first PD) by two-step immunoprecipitation using His6- and FLAG-tag of HBF-LYAR. The purified HBF-LYAR complexes were immunoprecipitated with anti-UBF (αUBF) (A) or anti-SPT5 (αSPT5) (B) as the second affinity bait (second PD). T-REx 293 cells treated with Dox were used as the control. Proteins were detected by immunoblotting using the antibodies indicated to the right of the panels. Molecular mass markers (kDa) are indicated to the left of the panels. The nuclear extract (NE, 10 μg) was used as a loading control. (C) Immunoblotting for proteins that associated with BRD2 or BRD4. HBF-BRD2-TO cells and FLAG-BRD4-TO cells were treated with Dox for 24 h, and then the nuclear extracts were subjected to a two-step immunoprecipitation (IP) with anti-His6 and anti-FLAG. T-REx 293 cells treated with Dox were used as the control. Proteins that associated with HBF-BRD2 or FLAG-BRD4 were detected by immunoblotting using the antibodies indicated to the right of the panels. β-actin was used as the loading control. (D) Immunoblotting for HBF-LYAR-associated proteins upon the knockdown of BRD4. HBF-LYAR-TO cells were treated with an siRNA specific for BRD4 for 72 h. After induction with Dox, the nuclear extracts were subjected to two-step immunoprecipitation (IP) using His6- and FLAG-tag of HBF-LYAR.
