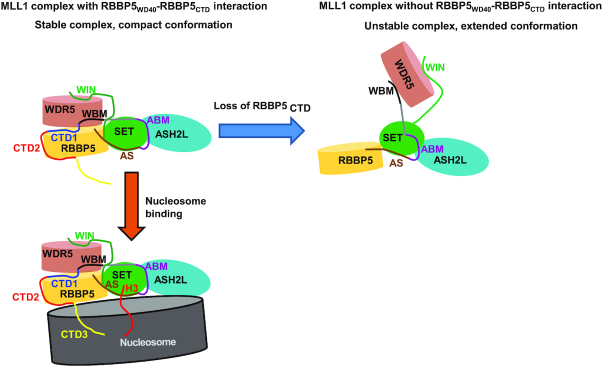
Figure 7.
The model for the role of RBBP5CTD in regulating MLL1 complex. The interaction between RBBP5WD40 and RBBP5CTD facilitates the formation of a compact MLL1 complex with correct coordination of the structural elements required for methylation. The loss of RBBP5CTD generates an extended complex with low catalytic efficiency. A vertebrate-specific RBBP5CTD3 can bind to nucleosome DNA and increases the association of the MLL1 complex with nucleosomes, thereby promoting methylation of nucleosomal H3.