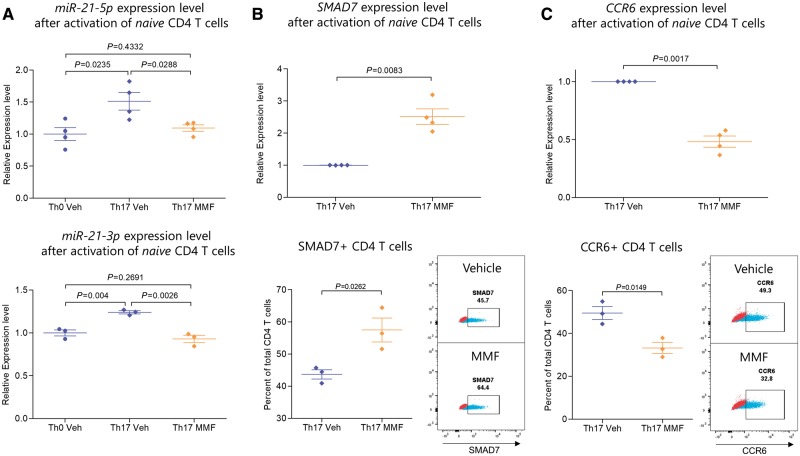
Figure 6.
FAE treatment reduced miR-21 and CCR6 expression and increased SMAD7 expression of CD4 T cells under Th17 polarizing conditions. Naïve (CD45RO−CCR7+) CD4 T cells were isolated from human PBMCs by FACS and were stimulated with anti-CD3 and anti-CD28 coated beads and cultured either without polarization (Th0) or under Th17 polarizing conditions with or without 50 µM of MMF. The expression of mature miR-21 transcripts (miR-21-5p and miR-21-3p) was measured by qPCR, which was first normalized to the geometric mean of SNORD44, SNORD48 and RNU6 expression level and then to the average relative expression level of the Th0 condition from all human donors. CCR6 and SMAD7 transcript levels were first normalized to the geometric mean of ACTB, B2M and HPRT1 expression level and then to the relative expression level of the vehicle treated Th17 condition from each human donor. Protein expression was measured by flow cytometry. (A) MMF resulted in the inhibition of upregulation of both miR-21-5p and miR-21-3p in Th17 polarizing conditions and kept their expression at Th0 levels. (B) SMAD7, a previously-identified miR-21 target that is known to inhibit the TGF-beta signalling pathway, was upregulated by MMF treatment as measured by qPCR and flow cytometry. (C) MMF reduced the expression of CCR6 measured by qPCR as well as the percentage of CCR6+ CD4 T cells measured by flow cytometry. MMF = 50 µM of MMF; Veh = vehicle. Flow cytometry plots show a representative sample of either vehicle or MMF treated CD4 T cells in blue and the fluorescence minus one (FMO) control in red, which was used for gating the positive population.