Abstract
Gonadal sex determination is a complex genetic process by which an embryonic primordium is driven to form an ovary or a testis, which requires a delicate dosage balance involving many genes. Disruption in this molecular pathway can lead to differences of sex development (DSD). Although some genetic mechanisms leading to 46,XY DSD have been elucidated, little is known about copy-number variation (CNV) causing testicular or ovotesticular 46,XX DSD. We describe a 20-year natural history of a man with SRY-negative 46,XX who was born with atypical male external genitalia, aortic coarctation, and bilateral blepharophimosis-ptosis. The molecular study identified a de novo heterozygous 3-Mb 15q26.2 deletion, a gene-poor locus containing NR2F2, which encodes the nuclear receptor COUP-TFII that is highly expressed in ovary and cardiac arteries. Immunohistochemistry confirmed the low COUP-TFII expression on his ovotestis tissue. Monosomy of 15q26.2, encompassing the NR2F2 gene, may act as a Z-factor regulating the male sex determination negatively. This finding supports a novel type of CNV resulting in DSD in an individual who developed male puberty spontaneously.
Keywords: 15q26.2 deletion; differences of sex development; gene regulation; copy-number variation; transcription factor binding sites; 46,XX DSD
46,XX ovotesticular differences/disorders of sex development (DSD), formerly known as true hermaphroditism [1], comprise a spectrum of sex anatomy promoted by rare variants of sexually dimorphic gonadal genes [2]. The sex determination in humans is a complex signaling pathway, which requires a delicate dosage balance involving many genes, acting either synergistically or antagonistically [3]. Disruption of these molecular pathways can lead to DSD. Although some genetic mechanisms leading to 46,XY DSD have been elucidated, little is known about testicular or ovotesticular 46,XX DSD [2, 3]. Approximately 80% of all 46,XX testicular DSD cases are explained by the presence of the SRY gene [1]. Other rare gain-of-function mechanisms include duplications of SOX9 or its regulatory region [2], copy-number variations (CNV) disrupting the regulatory region SOX3 [4], and partial duplications of human chromosome 22q13, containing SOX10 [2]. Additionally, loss-of-function mutations in female sex-determining (putative pro-ovary) genes can also lead to 46,XX DSD. It has been described in mutations in RSPO1 and WNT4, causing testicular 46,XX DSD. Mutations in RSPO1 are associated with palmoplantar hyperkeratosis and the susceptibility to squamous cell carcinoma [2, 5]. Also, identical NR5A1 mutations have been found in two unrelated patients with testicular/ovotesticular 46,XX DSD [6]. More recently, heterozygous mutations in NR2F2 have been linked to testicular/ovotesticular 46,XX DSD in children with cardiac defects [7].
Although the above genetic events are associated with 46,XX males, there is still much to be learned for the full understanding of ovotesticular DSD. In addition, incomplete penetrance and intrafamilial phenotype variability have been described among DSD-gene mutation carriers [8]. Notwithstanding, some recognized chromosomal rearrangements had been associated with urogenital and gonadal phenotypes, two being located at 15q21 and 15q24.1. Herein, we describe the natural history of the entire puberty development of a 46,XX ovotesticular DSD man caused by a novel 3-Mb 15q26.2 deletion, containing the NR2F2 gene. This region may function as the Z-factor [9], in which it plays a fundamental role in ovary development, acting as a negative regulator of male sex determination.
1. Case Report
A. Clinical History
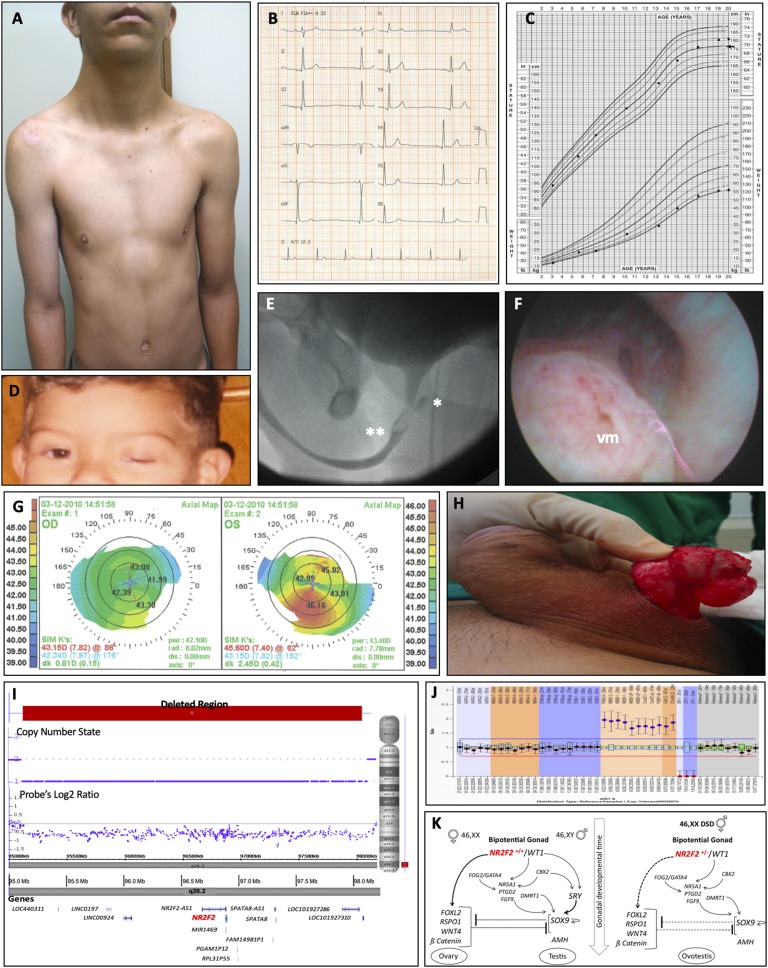
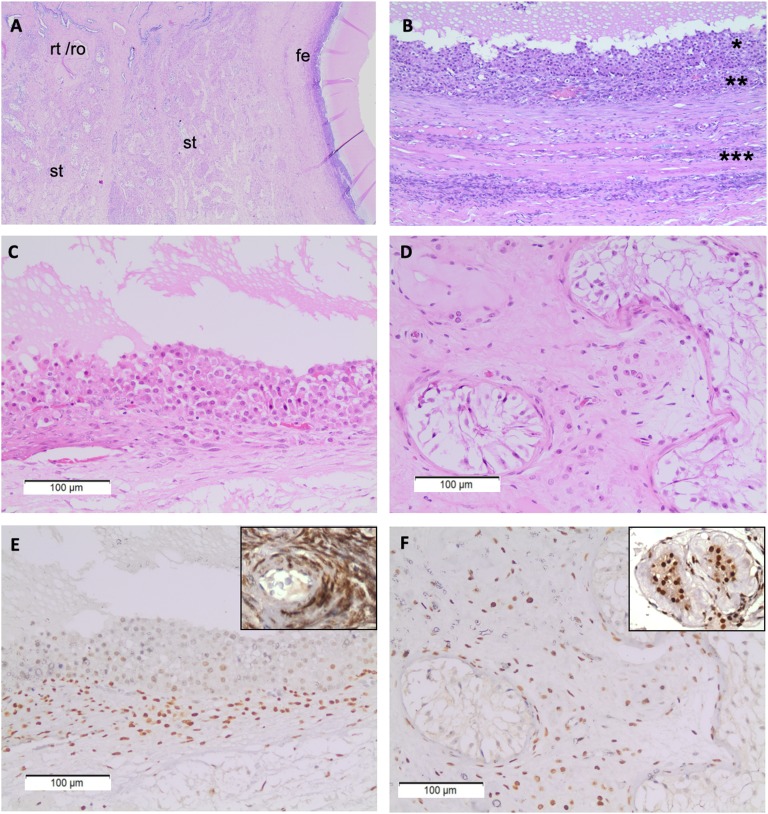
The reported patient is a 20-year-old man diagnosed with ovotestis. He was born from nonconsanguineous parents after an uneventful pregnancy, the third youngest boy in a healthy family. His lymphocyte culture revealed a 46,XX karyotype. At birth, a 3-cm penis with midshaft hypospadia, palpated inguinal gonads, and bilateral blepharophimosis-ptosis were detected. Low-set ears, webbed neck, pectus excavatum, and sinus bradycardia were noted (Fig. 1A and 1B). He presented low weight (5th percentile) but standard stature (75th percentile) for his age and surpassed parental target height (Fig. 1C). Asymmetric blepharophimosis-ptosis was documented by his parents (Fig. 1D). Urethrography showed a small prostatic utricle and a failure to fill the contrast in the posterior urethra (Fig. 1E). Cystoscopy identified a prominent bulging corresponding to verumontanum (Fig. 1F). Corneal topography diagnosed keratoconus when he was 11 years old (Fig. 1G). He underwent zetaplasty when he was 12 years old for correcting penile curvature that developed late after hypospadia repairs (Fig. 1H). At ∼14 years of age, he began spontaneous puberty, even though he presented with bilateral microorchidism (1 and 1.8 mL). He attained pubertal spurt and male secondary characteristics and no gynecomastia was evidenced. He developed an average penile length, male muscular distribution, and pubic hair, although scarce on the face (Fig. 1A). When he was 16 years old he underwent left gonadal biopsy and right gonadectomy because of a firm nodule in the right gonad diagnosed on histopathology as fibrotic tissue. Revised gonadal biopsies confirmed ovotesticular 46,XX DSD, comprising follicular epithelium mainly with granulosa cells in the ovarian part and Leydig cell hyperplasia and Sertoli cell-only tubules in the testicular part (Fig. 2A–2D). Gonadal axis tests are summarized in Table 1. He was assigned and raised as a boy, identified himself as male during childhood and adolescence, and has been living as heterosexual.
Figure 1.
Clinical and surgical investigation of a 46,XX man carrying heterozygous deletion at 15q26.2 encompassing NR2F2 gene. (A) Low-set posteriorly rotated ears, webbing of the neck, and mild pectus carinatum inferiorly with pectus excavatum superiorly. (B) Holter monitor indicating sinus bradycardia with the average measurement of 48 bpm. (C) Growth chart during clinical and surgical follow-up indicating his height (in/cm) and weight (ib/kg). (D) Asymmetric blepharophimosis-ptosis when he was 2 y old. (E) Urethrography showing the presence of a small prostatic utricle (*) and a failure to fill the contrast in the posterior urethra (**). (F) Cystoscopy identified a prominent lesion in verumontanum (vm) corresponding to the same position as the contrast-fill failure in urethrography. (G) Corneal topography indicating keratoconus when he was 11 y old during an investigation for low visual acuity. (H) Intraoperative macroscopic aspect of the right gonad obtained during zetaplasty performed for the treatment of penile curvature when he was 12 y old, which emerged in late postoperative of hypospadia repairs at 4 and 6 y old, showing a cystic structure close to the gonad that was identified as Müllerian remnant. (I) Chromosomal microarray result showing a de novo 3-Mb 15q26 deleted segment (red bar) using UCSC hg19 genome reference (horizontal gray bar) placed in a gene-poor region (transcripts; dark blue) including NR2F2 gene (red). (J) SALSA MLPA P185 Intersex® depicting patient’s probing for NR0B1 (DAX1) and CXorf21 on Xp21.2, SOX9 on 17q24.3, SRY and ZFY on Yp11.3, WNT4 on 1p36.12 and NR5A1 on 9q33, in addition to specific probes for the X- and Y-chromosomes. (K) Proposed NR2F2 signaling pathway for ovary and testis development. In XX embryos, this pathway occurs whether NR2F2 is in two functional copies. When carrying NR2F2 heterozygous (+/−) deletion, the upregulation of putative pro-ovary genes is diminished. Although in the absence of SRY, SOX9 is activated, but not sufficiently expressed to downregulate pro-ovary genes. In this peculiar context, neither pro-ovary genes are strong enough to inhibit the putative protestis SOX9, neither SOX9 is enough to inhibit pro-ovary genes, therefore, leading to ovotestis development.
Figure 2.
Immunohistochemical characterization of steroid hormone nuclear receptor COUP-TFII expression on the ovotestis gonad from 46,XX subject carrying a 3-Mb 15q26.2 heterozygous deletion. (A) Low-power scanning field showing left to right ovotesticular dysgenetic gonad containing testicular seminiferous tubules (st), merged rete testis/rete ovarii (rt/ro), and ovarian follicular epithelium (fe) with hematoxylin and eosin (H&E) staining. (B) Dysgenetic ovarian follicular epithelium showing granulosa (*) and theca (**) cell layers, surrounded by ovarian stroma (***) on H&E (200×). (C) Granulosa cells lining in a theca lutein cyst where no primary oocytes were found (H&E). (D) Seminiferous tubules are devoid from germinative cells and contain only Sertoli cells surrounded by some luteinized stromal cells (H&E). Weak nuclear immunostaining for COUP-TFII immunoperoxidase in granulosa (E) and Sertoli (F) cells fulfilling seminiferous tubules of ovotesticular tissue in comparison with ovary (from young woman/ 200×) and testis (elderly man / 400×) external control tissues used in the same immunostaining bath, which are zoomed in the upper-right-hand corner of E and F.
Table 1.
Gonadal Axis Hormone Measurements During 20-Year Follow-Up of a Man with Ovotesticular 46,XX Due to 15q26.2 Haploinsufficiency
| Period | Age (yr) | Total T ng/dL (male NR) | LH IU/L (NR) | FSH IU/L (NR) |
|---|---|---|---|---|
| Newborn | 66 d | 120 (<30) | NA | NA |
| During childhood | 2 | 30 (<30) | 0.52 (<0.10) | 3.0 (<0.3) |
| 3.2 | 22 (<30) | 0.90 (< 0.10) | 2.2 (<0.3) | |
| Very early to late adolescence | 11.7 | 127 (30–150) | NA | NA |
| 12.5 | 188 (30 –150) | 1.86 (<2.28) | 4.6 (0.30 –4.00) | |
| 13 | 202 (30–150) | 5.1 (0.31–5.29) | 6.9 (0.30 –4.00) | |
| 14.6 | 340 (241–827) | 4.8 (0.31–5.29) | 19.1 (0.40–7.40) | |
| 16 | 407 (241–827) | 32.4 (1.50–9.30) | 63.8 (0.40–7.40) | |
| 17.8 | 177 (241–827) | 39 (1.50 –9.30) | 91 (0.40–7.40) | |
| 19.7 | 122 (241– 827) | 43 (1.50 –9.30) | 97.7 (0.40–7.40) |
Normal range refers to the interval of basal levels in control subjects matched according to age and male sex. The conversion factor used: testosterone ng/dL × 0.034 for nmol/L.
Abbreviations: NA, not available; NR, normal reference; T, testosterone.
B. Genetic and Immunohistochemistry Analyses
Upon detailing his 46,XX karyotype, single nucleotide polymorphism-array analysis was performed and revealed a de novo 3-Mb deletion on 15q26: arr[GRCh37]15q26.2(95127653_98146649)×1, causing partial 15q monosomy (Fig. 1I). This region is an evolutionarily conserved locus among mammals and contains two genes (NR2F2 and SPATA8), three noncoding genes (NR2F2-AS1, SPATA8-AS1, and miR-1469), and three pseudogenes (PGAM1P12, RPL31P5, and FAM149B1P1) (Fig. 1I). The locus 15q26.2 also encompasses regulatory regions, containing more than 15,000 transcription factor binding (TFB) sites, among them, 282 to SRY, 147 to SOX5 and 103 to SOX9 (data not shown). PCR and Sanger sequencing from peripheral blood DNA were negative for common 46,XX DSD variants in SRY, RSPO1, SOX9, as well as NR2F2. MLPA revealed no CNV for WNT4, NR5A1, and SOX9 (Fig. 1J). Additionally, whole-exome sequencing performed in the proband and his parents resulted in no pathogenic DSD variants.
Once we ruled out SOX9, SOX3, WNT4, FOXL2, RSPO1, and NR2F2 missense mutations and that NR2F2 is missing in the 3-Mb 15q26 deletion, we further performed immunohistochemistry in the resected gonadal tissue and found lower COUP-TFII expression in granulosa-type cells and Sertoli cells compared with sex-cord stromal cells (Fig. 2E–2F).
2. Discussion
A de novo 3-Mb deletion on 15q26, encompassing NR2F2 gene and hundreds of sex-associated TFB sites, constitutes a novel genetic rearrangement in a 46,XX subject born with atypical male genitalia who developed all secondary male sexual characteristics. Examining his histopathology gonad thoroughly, we hypothesized that the ovotestis might have evolved to progressive gonadal fibrosis. Likely it may have begun slightly during the fetal period and then gradually increased up to the time of the midfinal period of puberty. The gradual testis atrophy leading to micro-orchidism must have resulted in Sertoli cells death, therefore, a decrease of inhibin B secretion and FSH increase as observed at the end of his adolescence. By compromising Leydig cells subsequently, the ongoing atrophy might have resulted in decreased testosterone synthesis and elevated LH level by a negative feedback loop.
This new 15q26.2 CNV implies NR2F2/COUP-TFII haploinsufficiency and is consistent with its diminished expression observed in ovotestis gonad, indicating its role in controlling DSD. Many recognized chromosomal rearrangements had been associated with urogenital and gonadal phenotypes [8]. In 2017, we reported the 15q26.2 CNV in association with 46,XX ovotesticular DSD, and cardiac phenotype [10]. Most CNVs are related to a loss-of-function mechanism, and few are related to upregulation of putative protestis factors in the developing 46,XX gonads as reported in SOX9 and SOX3 [8]. Other 46,XX testicular or ovotesticular DSDs result from mutations in pro-ovary/antitestis genes of the WNT4/RSPO1 signaling pathway, including loss-of-function mutations in WNT4 and homozygous RSPO1 mutations. Also, mutations in the FOXL2 gene are linked to ovary insufficiency and blepharophimosis-ptosis phenotype [8].
The verified NR2F2 heterozygous deletion can explain the genital ridge mesenchyme switch from an ovary to testis caused by the disruption of ovary-specific signaling, which would oppose testis differentiation. Zhao et al. (2017) recently showed that Nr2f2 (−/−) 46,XX mouse embryos developed mixed ductal mesenchyme, leading to both female and male reproductive tracts [11]. Our report is in agreement with Zhao’s active pro-ovarian mechanism and validates in human the hypothesis that the regression of Wolffian ducts in 46,XX embryos is actively driven by COUP-TFII by suppressing mesenchyme-epithelium crosstalk responsible for Wolffian maintenance [11]. It has been described that NR2F2 is expressed at the same time of WT1 in early gonadal embryogenesis. Therefore, our data are consistent with previous observations on the role of NF2R2 [7, 11]. We hypothesized that the dosage-sensitive loss of NR2F2 could release WT1 to trigger the expression of SOX9 through NR5A1 in the absence of SRY (Fig. 2K).
Moreover, the 3-Mb 15q26.2 deletion also containing putative protestis (SOX9, SOX3, and SOX10) TFB sites may impair sex dimorphic regulation of target genes in the bipotential gonad. This regulatory haploinsufficiency can lead to incomplete testicular development, as observed in our patient, once SOX9 partial activation is not sufficient to completely block alleged pro-ovary gene expression (Fig. 2K). Bashamboo et al. [7], applying exome sequencing on 46,XX SRY-negative individuals with unexplained virilization or testicular/ovotesticular DSD, identified heterozygous mutations in NR2F2 in three young children. They presented congenital heart disease, without palpable gonads, and two of them having blepharophimosis-ptosis-epicanthus inversus syndrome [7]. For that reason, the 15q26.2 region encompassing NR2F2 and hundreds of putative protestis TFB sites may function as a Z-factor region, being crucial for ovary development, as hypothesized in 1993 by McElreavey et al. [9].
Therefore, our data show that the locus 15q26.2 can be considered one of the Z-factor regions for the sex development in XX embryos because both the monosomy of this chromosomal region and the haploinsufficiency of NR2F2 are not capable of defining the full differentiation of either female or male gonads.
Acknowledgments
We thank Ilda Kunii for laboratory technical assistance, Professor Susan Lindsey for manuscript revision, and Professor Rui Maciel for sharing laboratory support.
Financial Support: This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP grants 2011/20747-8, 2012/00079-3, 2014/06570-6 and 2014/11572-8), CAPES and CNPq.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Glossary
Abbreviations:
- CNV
copy-number variation
- DSD
differences of sex development
- TFB
transcription factor binding
Contributor Information
Gianna Carvalheira, Email: carvalheira@unifesp.br.
Magnus R Dias-da-Silva, Email: mrdsilva@unifesp.br.
References and Notes
- 1. Berglund A, Johannsen TH, Stochholm K, Aksglaede L, Fedder J, Viuff MH, Main KM, Gravholt CH. Incidence, prevalence, diagnostic delay, morbidity, mortality and socioeconomic status in males with 46,XX disorders of sex development: a nationwide study. Hum Reprod. 2017;32(8):1751–1760. [DOI] [PubMed] [Google Scholar]
- 2. Grinspon RP, Rey RA. Disorders of sex development with testicular differentiation in SRY-negative 46,XX individuals: clinical and genetic aspects. Sex Dev. 2016;10(1):1–11. [DOI] [PubMed] [Google Scholar]
- 3. Eggers S, Ohnesorg T, Sinclair A. Genetic regulation of mammalian gonad development. Nat Rev Endocrinol. 2014;10(11):673–683. [DOI] [PubMed] [Google Scholar]
- 4. Grinspon RP, Nevado J, Mori Alvarez ML, Del Rey G, Castera R, Venara M, Chiesa A, Podestá M, Lapunzina P, Rey RA. 46,XX ovotesticular DSD associated with a SOX3 gene duplication in a SRY-negative boy. Clin Endocrinol (Oxf). 2016;85(4):673–675. [DOI] [PubMed] [Google Scholar]
- 5. Elzaiat M, Todeschini AL, Caburet S, Veitia RA. The genetic make-up of ovarian development and function: the focus on the transcription factor FOXL2. Clin Genet. 2017;91(2):173–182. [DOI] [PubMed] [Google Scholar]
- 6. Igarashi M, Takasawa K, Hakoda A, Kanno J, Takada S, Miyado M, Baba T, Morohashi KI, Tajima T, Hata K, Nakabayashi K, Matsubara Y, Sekido R, Ogata T, Kashimada K, Fukami M. Identical NR5A1 missense mutations in two unrelated 46,XX individuals with testicular tissues. Hum Mutat. 2017;38(1):39–42. [DOI] [PubMed] [Google Scholar]
- 7. Bashamboo A, Eozenou C, Jorgensen A, Bignon-Topalovic J, Siffroi JP, Hyon C, Tar A, Nagy P, Sólyom J, Halász Z, Paye-Jaouen A, Lambert S, Rodriguez-Buritica D, Bertalan R, Martinerie L, Rajpert-De Meyts E, Achermann JC, McElreavey K. Loss of function of the nuclear receptor NR2F2, encoding COUP-TF2, causes testis development and cardiac defects in 46,XX children. Am J Hum Genet. 2018;102(3):487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yatsenko SA, Witchel SF. Genetic approach to ambiguous genitalia and disorders of sex development: what clinicians need to know. Semin Perinatol. 2017;41(4):232–243. [DOI] [PubMed] [Google Scholar]
- 9. McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc Natl Acad Sci USA. 1993;90(8):3368–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carvalheira G, Moysés-Oliveira M, Ueta RM, Dias da Silva MR, Melaragno MI. SRY-negative 46,XX male with ovotesticular disorder of sex development and cardiac defects due to a 3-Mb deletion in 15q [abstract]. Mol Cytogenet. 2017;10(Suppl 1):37.29075328 [Google Scholar]
- 11. Zhao F, Franco HL, Rodriguez KF, Brown PR, Tsai MJ, Tsai SY, Yao HH. Elimination of the male reproductive tract in the female embryo is promoted by COUP-TFII in mice. Science. 2017;357(6352):717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]