Abstract
Background
Interactions between the endothelium and infected erythrocytes play a major role in the pathogenesis of falciparum malaria, with microvascular dysfunction and parasite sequestration associated with worsening outcomes. The glycocalyx is a carbohydrate-rich layer that lines the endothelium, with multiple roles in vascular homeostasis. The role of the glycocalyx in falciparum malaria and the association with disease severity has not been investigated.
Methods
We prospectively enrolled Indonesian inpatients (aged ≥18 years) with severe (SM) or moderately severe (MSM) falciparum malaria, as defined by World Health Organization criteria, and healthy controls (HCs). On enrollment, blood and urine samples were collected concurrently with measurements of vascular nitric oxide (NO) bioavailability. Urine was assayed for glycocalyx breakdown products (glycosaminoglycans) using a dimethylmethylene blue (GAG-DMMB) and liquid chromatography-tandem mass spectrometry (GAG-MS) assay.
Results
A total of 129 patients (SM = 43, MSM = 57, HC=29) were recruited. GAG-DMMB and GAG-MS (g/mol creatinine) were increased in SM (mean, 95% confidence interval: 3.98, 2.44–5.53 and 6.82, 5.19–8.44) compared to MSM patients (1.78, 1.27–2.29 and 4.87, 4.27–5.46) and HCs (0.22, 0.06–0.37 and 1.24, 0.89–1.59; P < 0.001). In SM patients, GAG-DMMB and GAG-MS were increased in those with a fatal outcome (n = 3; median, interquartile range: 6.72, 3.80–27.87 and 12.15, 7.88–17.20) compared to survivors (n = 39; 3.10, 0.46–4.5 and 4.64, 2.02–15.20; P = 0.03). Glycocalyx degradation was significantly associated with parasite biomass in both MSM (r = 0.48, GAG-DMMB and r = 0.43, GAG-MS; P < 0.001) and SM patients (r = 0.47, P = 0.002 and r = 0.33, P = 0.04) and inversely associated with endothelial NO bioavailability.
Conclusions
Increased endothelial glycocalyx breakdown is associated with severe disease and a fatal outcome in adults with falciparum malaria.
Keywords: Plasmodium falciparum, severe malaria, endothelium, glycocalyx
In a prospective study of Indonesian adults with falciparum malaria, glycocalyx breakdown was associated with impaired endothelial nitric oxide bioavailability, increased parasite biomass, endothelial activation, disease severity, and risk of death.
(See the Editorial Commentary by Georgiadou and Cunnington on pages 1721–3.)
Malaria remains a major cause of morbidity and mortality, with 216 million symptomatic cases and 445 000 deaths in 2016, mainly from Plasmodium falciparum infections [1]. Approximately 15% of adults with severe falciparum malaria (SM) die despite treatment with the most effective antiparasitic therapy, intravenous artesunate [2]. Further improvements in outcomes require better supportive care or adjunctive agents to attenuate the pathogenic mechanisms of severe disease.
Host–pathogen interactions between the vascular endothelium and P. falciparum–infected erythrocytes play a major role in the pathogenesis of SM [2, 3]. Parasitized erythrocytes express erythrocyte membrane proteins that cytoadhere to endothelial receptors on capillaries and post-capillary venules, resulting in parasite sequestration [3]. Clinical studies have demonstrated dysregulated vascular homeostasis in malaria, with decreased vascular nitric oxide (NO) bioavailability and increased endothelial activation in proportion to disease severity [4, 5]. In SM, parasite sequestration and endothelial dysfunction are independent predictors of death [5, 6], each contributing to impaired microcirculatory flow, tissue hypoxia, and organ dysfunction [7].
The glycocalyx consists of a functional carbohydrate- and protein-rich layer that overlies endothelial cells [8, 9]. It is hydrophobic and consists of transmembrane anchoring proteins, including syndecans and glypicans, covalently linked to glycosaminoglycan side chains such as heparan sulfate (HS) and chondroitin sulfate (CS), with embedded proteins including albumin [8, 9]. The glycocalyx has multiple roles in vascular physiology, functioning as a transducer of shear stress to regulate endothelial cell NO release [10] and as a barrier to prevent translocation of intravascular fluid and proteins into the interstitial compartment; it also attenuates leukocyte- and platelet-binding to the endothelium during inflammation [9]. Glycocalyx breakdown is a key process in the pathogenesis of sepsis, inflammation, and ischemic-reperfusion injury [9] and has been associated with disease severity in dengue [11] and an increased risk of acute kidney injury (AKI) in septic shock [12].
Glycocalyx breakdown results in decreased vascular NO [10] and increased exposure of the endothelial receptors that mediate cytoadherence of infected erythrocytes [13]. In vitro, increased glycocalyx thickness decreases binding by P. falciparum–infected erythrocytes [14], and murine malaria models have shown almost complete loss of the glycocalyx in experimental cerebral malaria compared to uncomplicated malaria [15, 16]. However, the role of the glycocalyx in clinical malaria is not known. We measured glycocalyx breakdown products in Indonesian adults with or without severe malaria. We hypothesized glycocalyx breakdown is increased in proportion to malaria disease severity, associated with parasite sequestration, and inversely related to endothelial NO bioavailability.
METHODS
This prospective, observational study was conducted at Mitra Masyarakat Hospital in Papua, Indonesia, an area with unstable transmission of P. falciparum. Ethical approval was obtained from the Health Research Ethics Committees of the National Institute of Health Research and Development, Indonesia, and the Menzies School of Health Research, Australia. Written informed consent was obtained from patients or attending relatives in Indonesian or a local language.
Participants
Adults aged ≥18 years were enrolled from the emergency department or outpatient clinics, as previously reported [4], and classified as severe malaria (SM), moderately severe malaria (MSM), or healthy controls (HC) according to the following criteria: SM, microscopic diagnosis of P. falciparum with ≥1 World Health Organization (WHO) criteria for SM (see Supplementary Materials); MSM, fever or history of fever in the past 48 hours with >1000 asexual P. falciparum parasites/µL, requiring inpatient parental therapy due to inability to tolerate oral treatment, and no WHO warning or SM criteria; HC, defined as unrelated hospital visitors with no history of fever in the last 48 hours, no parasitemia, and no concurrent illness. Exclusion criteria included being pregnant or breastfeeding; received parental antimalarial for longer than 18 hours; mixed P. falciparum/P. vivax infections; diabetes mellitus and known cardiac, renal, or hepatic disease; concurrent infections; and hemoglobin <60 g/L. Patients were treated according to prevailing Indonesian National Malaria Management Guidelines.
Study Procedures
Clinical Observations
Upon enrollment, clinical details were recorded in standardized data collection forms, venous blood and urine were collected, and endothelial function was measured as described below.
Laboratory Methods
Hemoglobin and leukocyte counts were measured using a coulter counter (T890; Beckman Coulter); biochemistry, acid-base parameters, and lactate were measured using a handheld i-STAT biochemical analyzer. Parasite counts were determined by examination of Giemsa-stained blood films (see Supplementary Material). Plasma was separated within 30 minutes of venesection by centrifugation, urine was collected and centrifuged, and both were stored at –80ºC. Plasma concentrations of syndecan-1, endothelial activation markers (angiopoietin-2, intercellular adhesion molecule 1 (ICAM-1), and E-selectin), cell-free hemoglobin, sphingosine-1-phosphate, and parasite biomass (plasma histidine-rich protein-2 [HRP2]) were measured using enzyme-linked immunosorbent assay, as previously reported [4, 5, 17].
Urinary glycosaminoglycans (GAG) are glycocalyx breakdown products that are stable over extended periods of time and conditions, including freezing, and were assessed using 2 methods [12, 18] (detailed in full in the Supplementary Materials).The first was a dimethylmethylene blue (DMMB) colorimetric assay that measured total urinary sulfated GAG as previously described [12, 19]. The second measured total and individual GAG including HS, dermatan sulfate (DS), and CS by an isotope-dilution ultraperformance liquid chromatography–electrospray ionization tandem mass-spectrometry (UPLC-MS/MS) method, as described previously [20]. Urinary creatinine levels were determined by the alkaline picrate method, and the urine GAG concentrations were normalized to creatinine levels [20, 21].
Endothelial Function
Vascular NO bioavailability was measured noninvasively using reactive hyperemia-peripheral arterial tonometry, a validated methodology described in detail previously that is ≥50% dependent on NO release [4] (see Supplementary Materials).
Statistical Methods
Intergroup differences for continuous variables were compared by analysis of variance or the Kruskal-Wallis test. Post-hoc multiple pairwise comparisons were used to compare SM, MSM, and HCs with adjustment using the Sidak method [22]. Pearson or Spearman methods were used to determine correlation coefficients for continuous variables. Multiple stepwise linear regression was conducted to adjust for confounding variables that could influence the association between markers of disease severity, endothelial activation/function, with GAG levels. Variables hypothesized to contribute to disease severity and endothelial dysfunction were included. Partial correlation coefficients were calculated, adjusting for disease severity. To measure the prognostic utility of continuous variables, the area under the receiver-operating curve (AUROC; 95% confidence interval [CI]) were calculated. Statistical analysis was conducted with Stata v14. A 2-sided value of P < 0.05 was considered significant.
RESULTS
Patients
In total, 129 adults were enrolled in 2005–2006, 43 SM, 57 MSM, and 29 HCs. Clinical features of these patients have been described previously [4]. In SM patients, the common complications were cerebral malaria (24/43, 56%), hyperbilirubinemia (20/43, 47%), and severe AKI (13/43, 30%). Twenty (47%) patients had 1 severity criterion, 14 (32%) had 2, 5 (12%) had 3, and 4 (9%) had 4. In SM patients, 27 (63%) received intravenous artesunate and 16 (43%) received quinine. Among MSM patients, 55 (97%) were treated with intravenous quinine and 2 with artesunate. There were 3 (7%) deaths in the SM group and none in those with MSM. Baseline characteristics of the patients are summarized in Table 1.
Table 1.
Baseline Demographic Characteristics, Clinical Features, and Vital Signs Among Patient Groups
| Patient Characteristics | Healthy Control Group (n = 29) |
Moderately Severe Malaria (n = 57) |
Severe Malaria (n = 43) |
P Valuea |
|---|---|---|---|---|
| Age, y | 27 (18–35) | 27 (18–56) | 25 (18–56) | .02 |
| Male sex, no. (%) | 16 (64) | 39 (68) | 33 (72) | .2 |
| Fever duration before admission (days) | Not applicable | 2 (1–5) | 4 (1–7) | .5 |
| Weight, kg | 55 (45–71) | 57 (42–73) | 57 (36–70) | < .001 |
| Temperature, °C | 35.5 (35–36.7) | 36.7 (35–40.2) | 37.2 (34.6–39.4) | < .001 |
| Blood pressure, mm Hg | ||||
| Systolic | 131 (106–158) | 112 (89–136) | 114 (74–141) | < .001 |
| Diastolic | 76 (59–100) | 67 (49–86) | 62 (39–89) | < .001 |
| Pulse rate, beats/min | 69 (44–104) | 84 (56–118) | 92 (59–132) | < .001 |
| Respiratory rate, breaths/min | 20 (18–24) | 24 (18–34) | 28 (16–46) | < .001 |
All results are median (range), unless otherwise specified.
aBy analysis of variance or Kruskal-Wallis test, comparing the healthy control, moderately severe malaria, and severe malaria groups.
Glycocalyx Breakdown Products and Disease Severity
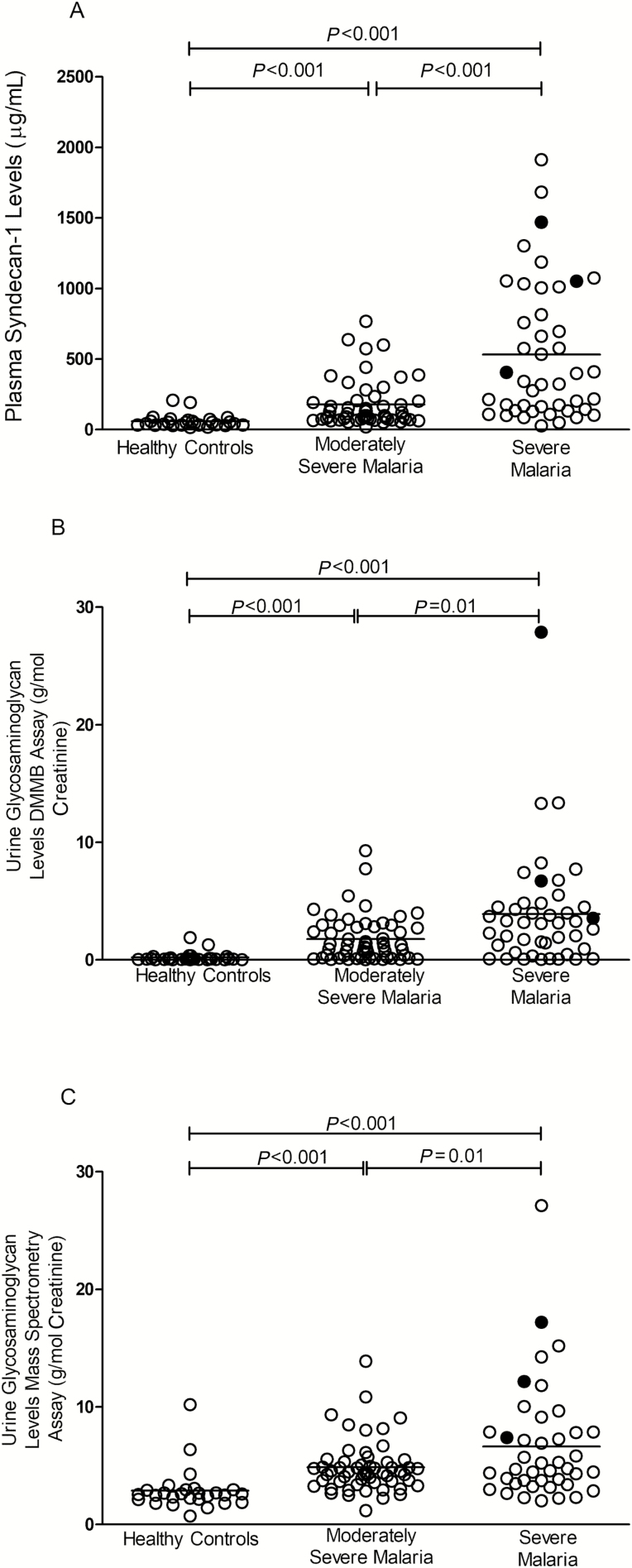
Plasma concentrations of syndecan-1 and urinary concentrations of total GAG, measured by both DMMB and LC-MS/MS methods, were higher in SM compared to MSM and HC (P < .001; Table 2, Figure 1A–1C). In SM, there were significant increases in syndecan-1 (P = .03) and GAG-MS (P = .04) with increasing number of complications. In patients with severe AKI, levels of syndecan-1 (P = .02) and GAG-MS (P = .03) were significantly elevated compared to those without, with both syndecan-1 and GAG-MS levels being significantly higher than the MSM group. In a multivariable model of glycocalyx breakdown products, biomarkers of disease severity, and endothelial activation, variables hypothesized to contribute to disease severity and found to be significantly different (P < .05) between SM and MSM (Table 2) were included. Syndecan-1, angiopoietin-2, and HRP all remained significantly associated with increased risk of SM.
Table 2.
Baseline Hematological, Biochemical, and Endothelial Functions and Laboratory Tests Among Patient Groups
| Laboratory and Physiological Characteristics | Healthy Control (n = 29) |
Moderately Severe Malaria (n = 57) |
Severe Malaria (n = 43) |
P Valuea |
|---|---|---|---|---|
| White blood cell count, ×103 cells/µL (normal range, 4.0–10.03 cells/µL) | ND | 6.2 (2.3–11.7) | 8.6 (3.2–17.3) | < .001 |
| Hemoglobin, g/dL (normal range, 12.5–17.5 g/dL) | ND | 12.2 (7.5–16.7) | 10.9 (4.2–15.6) | .003 |
| Creatinine level, mmol/L (normal range, 45–110 mmol/L) | ND | 86 (42–174) | 170 (51–1193) | < .001 |
| Lactate level, mmol/L (normal range, 0.5–1 mmol/L) | ND | 1.2 (0.6–3.2) | 2.8 (0.65–8.3) | < .001 |
| Parasite density, parasite/µL geometric mean (95% CI) | 0 | 13 556 (9648–19074) | 27 327 (17 715–38 914) | .001 |
| Plasma histidine-rich protein-2 concentration, ng/mL | Not applicable | 226 (2–6598) | 3546 (2.5–44 717) | < .001 |
| Cell-free hemoglobin, µM | 1.4 (1.1–2.7) | 3.4 (2.8–4.2) | 6.8 (4.8–8.8) | < .001 |
| Soluble ICAM-1, mean (95% CI), pg/mL | 335 (296–443) | 575 (513–635) | 1072 (792–1352) | < .001 |
| Soluble E-selectin, mean (95% CI), pg/mL | ND | 104 (91–117) | 149 (102–195) | .03 |
| Plasma angiopoietin 2, mean (95% CI), pg/mL | 2661 (1796–3525) | 6238 (5033–7443) | 15916 (11745–20088) | < .001 |
| Reactive hyperemia-peripheral arterial tonometry index, mean (95% CI) | 1.84 (1.66–2.02) | 1.76 (1.65–1.88) | 1.44 (1.36–1.51) | < .001 |
| Sphingosine-1-phosphate, mean (95% CI), µM | 4.4 (3.3–5.8) | 3.7 (3.2–4.3) | 2.5 (1.9–3.0) | .005 |
| Syndecan-1 mean (95% CI), ng/mL | 58.5 (40.8–76.2) | 178.2 (131.9–224.5) | 532.1 (384.2–679.9) | < .001 |
| Urine glycosaminoglycans, dimethylmethylene blue assay, g/mol creatinine (95% CI) | 0.22 (0.06–0.37) | 1.78 (1.27–2.29) | 3.98 (2.44–5.53) | |
| Urine glycosaminoglycan,b mass spectrometry assay, g/mol creatinine | 2.87 (2.16–3.57) | 4.87 (4.27–5.46) | 6.82 (5.19–8.44) | < .001 |
| Urine chondroitin sulfate, g/mol creatinine | 1.24 (0.89–1.59) | 3.23 (2.72–3.75) | 4.55 (3.15–5.95) | < .001 |
| Urine dermatan sulfate, g/mol creatinine | 0.91 (0.68–1.16) | 0.84 (0.75–0.94) | 0.73 (0.59–0.86) | .18 |
| Urine heparan sulfate, g/mol creatinine | 0.67 (0.57–0.76) | 0.81 (0.72–0.89) | 1.53 (1.22–1.7) | < .001 |
All results are median (range), unless otherwise specified.
Abbreviations: CI, confidence interval; ICAM-1, intercellular adhesion molecule 1; ND, not done.
aBy analysis of variance or Kruskal-Wallis test, comparing the healthy control, moderately severe malaria, and severe malaria groups.
bTotal concentration of chondroitin sulfate, dermatan sulfate, and heparan sulfate determined by ultraperformance liquid chromatography–electrospray ionization tandem mass-spectrometry method.
Figure 1.
A, Plasma syndecan-1 levels measured in Indonesian adults who were enrolled as healthy controls and with moderately severe malaria or severe malaria. B, Urinary glycosaminoglycan levels measured by dimethylmethylene blue colorimetric assay in Indonesian adults who were enrolled as healthy controls and with moderately severe malaria or severe malaria. C, Urinary glycosaminoglycan levels (sum of chondroitin sulfate, dermatan sulfate, and heparan sulfate) measured by a mass spectrometric method assay in Indonesian adults who were enrolled as healthy controls and with moderately severe malaria or severe malaria. Lines indicate mean value. P < .001 by analysis of variance. ○, survivors; ●, nonsurvivors. Abbreviation: DMMB, dimethylmethylene blue.
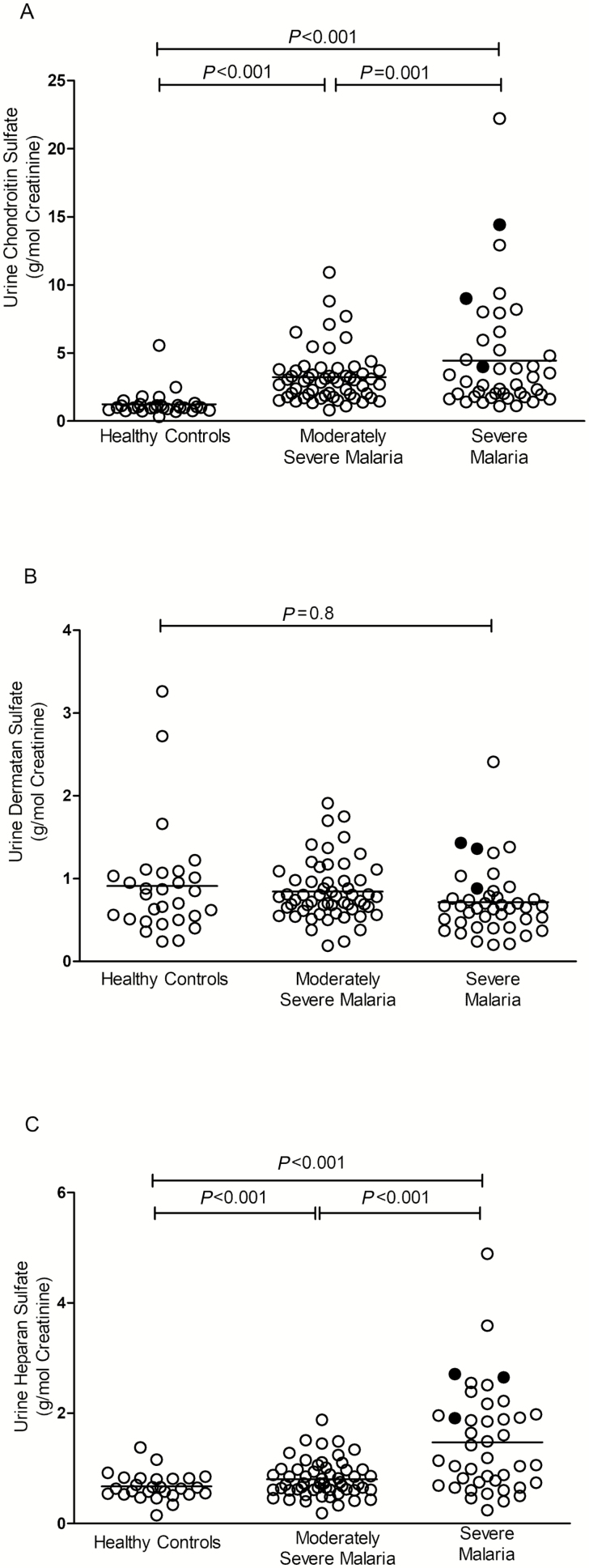
For individual GAG components, CS and HS were increased in SM compared to MSM patients and HCs (P < .001; Table 2, Figures 2A and 2C) and in SM patients with AKI compared to those without, however, there was no difference in DS (Table 2, Figure 2B). HS and CS products are major components of the endothelial glycocalyx, while only small amounts of DS are present in the endothelium [23, 24].
Figure 2.
A, Urinary chondroitin sulfate levels measured by a mass spectrometric method assay in Indonesian adults who were enrolled as healthy controls and with moderately severe malaria or severe malaria. B, Urinary dermatan sulfate levels measured by a mass spectrometric method assay in Indonesian adults who were enrolled as healthy controls and with moderately severe malaria or severe malaria. C, Urinary heparan sulfate levels measured by a mass spectrometric method assay in Indonesian adults who were enrolled as healthy controls and with moderately severe malaria or severe malaria. Lines indicate mean value. P < .001 by analysis of variance. ○, survivors; •, nonsurvivors.
There were significant associations between syndecan-1 and GAG-DMMB (r = 0.42, P < .001), GAG-MS (r = 0.45, P < .001), CS (r = 0.42, P < .001), and HS (r = 0.5, P < .001), but not with DS. To exclude the production of glycocalyx breakdown products from the parasite-infected erythrocytes in malaria patients, the DMMB assay was used to test supernatant media from in vitro cultures of P. falciparum. The culture supernatants from uninfected red blood cells (RBCs) and P. falciparum–infected RBC cultures did not contain GAG (data not shown).
Glycocalyx Breakdown Products and Biomarkers of Severity
There were significant differences in markers of disease severity between the SM and MSM patients. Venous lactate levels, peripheral parasitemia, and parasite biomass (HRP2) were higher in SM compared to MSM (Table 2).
After controlling for disease severity, parasite biomass as measured by HRP2 was correlated with syndecan-1 (partial correlation coefficient [pcorr] = 0.29, P = .006) and GAG concentrations determined by DMMB (pcorr = 0.39, P < .001) and MS (pcorr = 0.32, P = .002; Table 3). These correlations remained significant after adjusting for confounding factors, including age, sex, weight, ethnicity, severity, ICAM-1, angiopoietin-2, and endothelial function. Peripheral parasitemia also correlated with syndecan-1 (pcorr = 0.24, P = .03), GAG-DMMB (pcorr = 0.27, P = .01; Table 3), and GAG-MS (pcorr = 0.31, P = .002; Table 3), remaining significant after adjusting for confounders.
Table 3.
Partial Correlation Coefficients for Biomarkers of Severity and Endothelial Function/Activation in 100 Severe Malaria and Moderately Severe Malaria Patients Adjusting for Disease Severity
| Glycosaminoglycans Assay | Biomarker | Correlation | P Value | Degree of Freedom |
|---|---|---|---|---|
| Syndecan-1 | HRP2 | 0.29 | .006 | 98 |
| Parasitemia | 0.24 | .002 | 94 | |
| Cell-free hemoglobin | 0.27 | .01 | 94 | |
| ICAM-1 | 0.46 | <.001 | 94 | |
| E-selectin | 0.15 | .15 | 94 | |
| Angiopoietin-2 | 0.48 | <.001 | 94 | |
| RH-PAT | −0.1 | .51 | 94 | |
| Sphingosine-1-phosphate | −0.13 | .13 | 87 | |
| Glycosaminoglycans; dimethylmethylene blue | HRP2 | 0.39 | <.001 | 93 |
| Parasitemia | 0.27 | .01 | 99 | |
| Cell-free hemoglobin | 0.23 | .04 | 99 | |
| ICAM-1 | 0.35 | <.001 | 99 | |
| E-selectin | 0.26 | .02 | 99 | |
| Angiopoietin-2 | 0.31 | .002 | 99 | |
| RH-PAT | −0.30 | .003 | 99 | |
| Sphingosine-1-phosphate | −0.21 | .08 | 93 | |
| Glycosaminoglycans; liquid chromatography-tandem mass spectrometry | HRP2 | 0.32 | .002 | 93 |
| Parasitemia | 0.31 | .002 | 99 | |
| Cell-free hemoglobin | 0.16 | .09 | 99 | |
| ICAM-1 | 0.27 | .009 | 99 | |
| E-selectin | 0.33 | .001 | 99 | |
| Angiopoietin-2 | 0.18 | .1 | 99 | |
| RH-PAT | −0.30 | .004 | 99 | |
| Sphingosine-1-phosphate | −0.32 | .008 | 93 |
Abbreviation: HRP2, plasma histidine-rich protein-2; ICAM-1, intercellular adhesion molecule 1; RH-PAT, reactive hyperemia-peripheral arterial tonometry.
Cell-free hemoglobin, a marker of disease severity [4, 5, 17] and oxidative stress in falciparum malaria [25], was associated with syndecan-1 (pcorr = 0.27, P = .01) and GAG-DMMB (pcorr = 0.23, P = .03; Table 3), but not GAG-MS (pcorr = 0.16, P = .09; Table 3), remaining significant after controlling for disease severity.
Sphingosine-1-phosphate mediates glycocalyx integrity and is reduced in malaria [26, 27]. There was an inverse association between sphingosine-1-phosphate with GAG-DMMB (pcorr = –0.32, P = .008) and GAG-MS (pcorr = –0.21, P = .08) levels (Table 3), but not syndecan-1.
At the time of enrollment, there was a significant association between plasma creatinine and syndecan-1 (pcorr = 0.58, P = .002), but not with urinary GAG-DMMB or GAG-MS. There was no association between lactate concentration and syndecan-1 or urine GAG-DMMB or GAG-MS in all malaria patients after controlling for disease severity.
Glycocalyx Breakdown Products and Endothelial Function/Activation
Endothelial activation markers, including ICAM-1, E-selectin, and angiopoietin-2, were elevated in SM compared to MSM, and endothelial function decreased with increased disease severity (Table 2).
There were significant associations between ICAM-1 with syndecan-1 (pcorr = 0.46, P < .001) and GAG (pcorr = 0.35, P < .001 [DMMB] and pcorr = 0.27, P = .009 [MS]; Table 3). E-selectin was significantly associated with GAG (pcorr = 0.26, P = .02 [DMMB] and pcorr = 0.33, P = .001 [MS]; Table 3), but not syndecan-1. All of these remained significant in multivariable analysis adjusting for factors known to affect ICAM-1 and E-selectin as mentioned previously. There was also a significant association between angiopoietin-2 and syndecan-1 (pcorr = 0.48, P < .001) and GAG-MS (pcorr = 0.31, P = .002), but not GAG-DMMB (Table 3) methods. This remained significant after adjusting for factors hypothesized to affect angiopoietin-2 [5]. Conversely, endothelial function inversely correlated with GAG levels (pcorr = –0.30, P = .003 [DMMB] and pcorr = –0.30, P = .004 [MS]; Table 3), but not syndecan-1. This association remained significant after adjustment for confounders.
Glycocalyx Breakdown Products and Mortality
In the SM group, there were increased levels of total urinary GAG in fatal cases measured by DMMB (median [range] 6.72 [3.8–27.87]) and MS (12.15 [7.88–17.2]) assays compared to survivors (3.1 [0.04–13.1] [GAG-DMMB], and 4.64 [2.02–15.2] [GAG-MS]; P = .04 and P = .03, respectively). CS and HS were also increased in fatal vs nonfatal cases (P = .03 and P = .04, respectively). Syndecan-1 levels were increased in nonsurvivors (1051.8 [406.3–1470.1]) compared to survivors (332.4 [26.8–1913.4]), but this was not significant (P = .08).
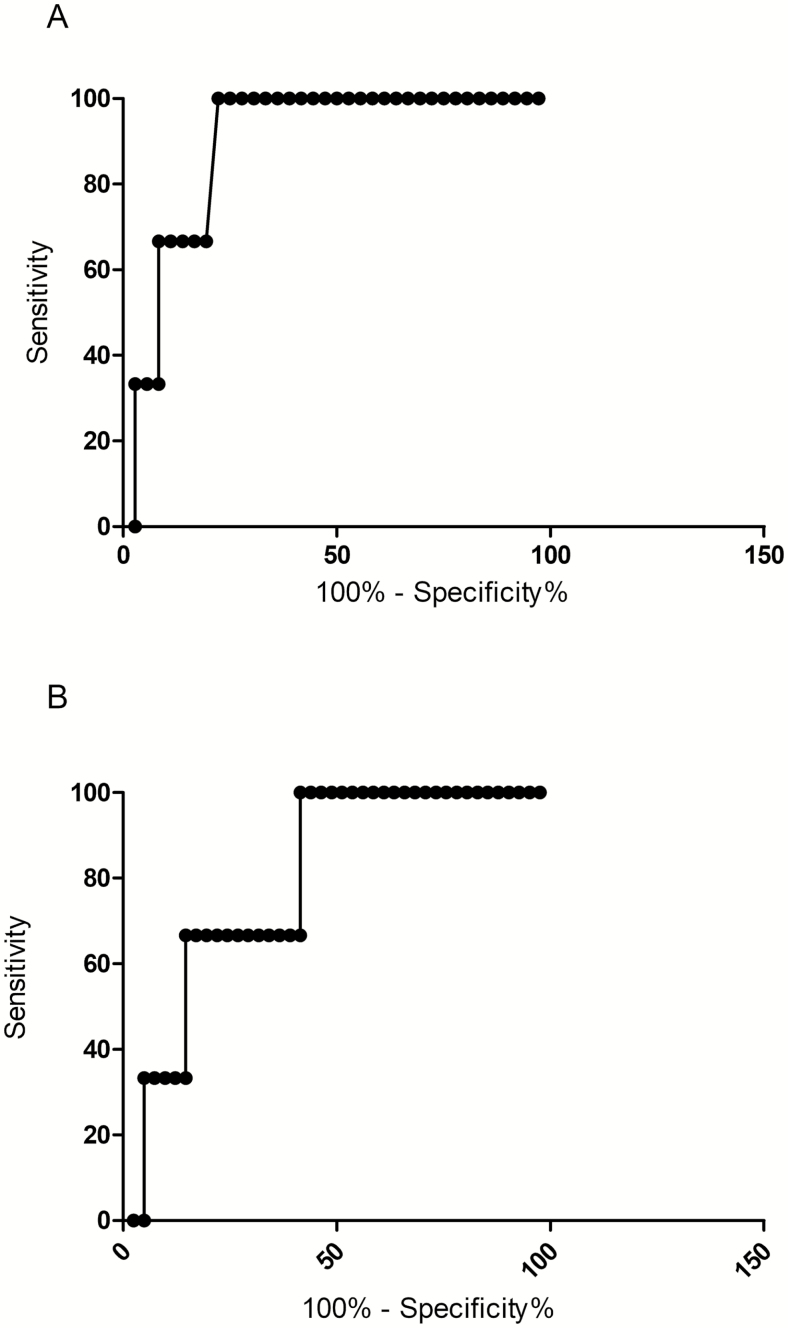
The AUROC was determined to assess the prognostic value of GAG and syndecan-1 concentrations in predicting a fatal outcome. The GAG-DMMB (AUROC, 0.84; 95% CI, 0.63–0.99), GAG-MS (AUROC, 0.89; 95% CI, 0.76–0.99), and syndecan-1 concentrations (AUROC, 0.78; 95% CI, 0.54–0.99) were comparable to other prognostic indicators such as angiopoietin-2 (AUROC, 0.83; 95% CI, 0.7–0.93) and parasite biomass (AUROC, 0.73; 95% CI, 0.53–0.98), which have been shown to be reliable predictors of a fatal outcome in adult malaria (Figure 3A and 3B).
Figure 3.
A, Nonparametric receiver operating curve to assess urinary glycosaminoglycan levels measured by mass spectrometry as a prognostic indicator for mortality in Indonesian adults with severe malaria. Area under the receiver-operating curve (AUROC), 0.89; 95% confidence interval [CI], 0.76–0.99. B, Nonparametric receiver operating curve to assess plasma syndecan-1 levels as a prognostic indicator for mortality in Indonesian adults with severe malaria. AUROC, 0.78; 95%, CI 0.54–0.99.
DISCUSSION
In adult falciparum malaria, endothelial glycocalyx breakdown was elevated in SM and associated with an increased risk of death. The inverse association between GAG levels and vascular NO bioavailability suggests glycocalyx damage impairs shear stress signaling and endothelial NO release. The association between endothelial activation markers and both syndecan-1 and GAG indicates glycocalyx damage increases the expression of endothelial receptors and parasite sequestration, as demonstrated by the relationship with parasite biomass. The significant role of glycocalyx breakdown in the pathogenesis of SM is highlighted by GAG levels being predictive of a fatal outcome. Glycocalyx degradation measured by both plasma and urinary markers, including a simple colorimetric method and the more complicated mass spectrometric analysis, were comparable.
The role of the endothelial glycocalyx in the pathogenesis of severe falciparum malaria has not been characterized previously. The endothelium plays a key role in the pathogenesis of malaria, and impaired vascular function is associated with severe disease [3, 7]. Similarly, the glycocalyx has multiple roles in vascular homeostasis [8, 9]. In vitro and in vivo animal studies have shown that several factors mediate glycocalyx breakdown [8, 9]. Studies in Asian adults and African children have found the angiogenic cytokine, angiopoietin-2 (Ang-2), to be a strong predictor of mortality in falciparum malaria [5, 28]. In vitro, Ang-2 increases glycocalyx breakdown [29], and the association between Ang-2 and syndecan-1/GAG levels in the current study suggests a similar role in malaria.
Oxidative stress is elevated in SM [30, 31] and can also mediate glycocalyx breakdown [9, 25]. Oxidative stress due to increased cell-free hemoglobin is associated with malaria severity, particularly risk of AKI [25], and glycocalyx breakdown is a major risk factor for AKI in sepsis [12]. Taken together, these studies suggest oxidative stress in critical illness may mediate glycocalyx damage and contribute to organ dysfunction. The associations between cell-free hemoglobin and syndecan-1/GAG in the current study suggest increased oxidative stress could contribute to glycocalyx breakdown and AKI in malaria. The lipid mediator, sphingosine-1-phosphate, protects against endothelial glycocalyx damage by inhibition of syndecan-1 shedding and increased glycocalyx synthesis and is decreased in malaria [26, 27]. The inverse association between sphingosine-1-phosphate and GAG in our study suggests decreased sphingosine-1-phosphate may also contribute to loss of glycocalyx integrity.
The glycocalyx is required for shear stress transduction and signaling for endothelial NO synthase (NOS)–mediated NO production by endothelial cells [10]. Impaired vascular NO bioavailability is associated with severe disease in falciparum malaria [4], with impairment due to multiple factors. These include lower concentrations of L-arginine, the substrate for NO synthase (NOS), decreased NOS expression [4, 32, 33], decreased levels of the NOS cofactor tetrahydrobiopterin [30, 31], increased asymmetrical dimethylarginine [34, 35], elevated arginase [4, 36], and quenching by cell-free hemoglobin, released during intravascular hemolysis [4, 17, 35]. Decreased endothelial function and concomitant increase in urinary GAG suggest that glycocalyx breakdown with reduced shear stress-signaling likely plays an additional role in decreasing vascular NO in malaria.
The glycocalyx functions as a “surface layer” to repel erythrocytes and shield endothelial receptors from intravascular cellular components. Decreased glycocalyx thickness could expose these receptors, thereby increasing binding with parasitized erythrocytes and worsening parasite sequestration. In vitro, increased glycocalyx thickness reduces binding between CD36-transfected cells with CD36-binding P. falciparum–infected erythrocytes [14]. In murine malaria models, greater glycocalyx degradation in the brain vasculature is seen in experimental cerebral malaria compared to uncomplicated disease models [15, 16]. In falciparum malaria, urinary GAG levels are associated with increased plasma ICAM-1 and E-selectin, which may reflect increased expression of endothelial adhesion-receptors due to glycocalyx shedding and loss of NO inhibition. This may result in increased parasite sequestration as evidenced by the associations between GAG levels with peripheral parasitemia and parasite biomass.
Glycocalyx damage could disrupt key vascular functions, leading to increased parasite sequestration, increased microvascular permeability, impaired microvascular flow, tissue hypoxia, and organ dysfunction. In this study, syndecan-1/GAG levels increased with worsening organ dysfunction and number of severity criteria. While syndecan-1/GAGs were increased in SM with or without AKI, reflecting pathogenic effects from systemic glycocalyx breakdown, the higher levels in those with AKI are consistent with a particular role in the pathogenesis of AKI, as seen in sepsis [12]. The effect of glycocalyx damage is further highlighted by the significantly increased GAG levels in patients with a fatal outcome compared to survivors in SM. Despite the small number of fatal cases in our study, GAG and syndecan-1 levels were predictive of mortality, with receiver operating characteristic curves comparable to other extensively validated biomarkers including parasite biomass and Ang-2 [5].
This study had several limitations. The observational nature of the design could not prove a causal relationship between glycocalyx breakdown with disease severity and outcome, but a mechanistic link is supported by the previous association of a syndecan-1 (SDC1)-gene polymorphism with protection from SM [37]. The small number of fatalities did not allow for adjustment of confounding factors. However, in separate multivariate models, both syndecan-1 and GAG remained correlated with parasite biomass after adjusting for multiple confounding factors. At the time of this study, tools to directly assess glycocalyx thickness in blood vessels were not available, and increased glycocalyx synthesis could result in no significant net change. Our measure of glycocalyx damage was based on GAG and syndecan-1 levels, which are indirect measures of breakdown products that originate mainly from endothelial cells, with minor contributions from other cells. In support of the endothelial origin of the GAGs in this study, HS and CS, the 2 most abundant GAG in the endothelial glycocalyx [38], were elevated in proportion to disease severity, but DS, which is not a major component of endothelial glycocalyx [38], was not. Finally, since we did not enroll children, our results cannot be extrapolated to pediatric malaria, and the pathogenic role of the glycocalyx needs to be further assessed in this population.
In summary, increased glycocalyx breakdown was associated with impaired NO bioavailability, increased disease severity, endothelial activation, parasite biomass, and mortality in adult falciparum malaria. A simple colorimetric assay to measure GAG levels may have potential utility as a biomarker for severe disease and death in falciparum malaria in resource-poor settings. Several agents can attenuate or repair glycocalyx damage in vitro or in animal studies [24, 39] and may have potential as adjunctive therapies for SM.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments.The authors thank all clinical and research staff involved in the studies at Rumah Sakit Mitra Masyarakat Hospital, Dr Emiliana Tjitra for support in the initial stage of the clinical study, Kim Piera for assistance, and the Papuan Health and Community Development Foundation.
Financial support. The study was supported by the National Health and Medical Research Council of Australia (grants 1132975, International Collaborative Research Grant 283321, HOTNORTH 1131932 [E. K.] and Fellowship 1135820 [N. A.]), the National Institutes of Health (grant 1R01 HL130763-01), the Durham VA Research Service (2772), the Wellcome Trust (ICRG ME928457MES), and the Singapore National Medical Research Council (award to T. W. Y., CSA INV 15nov007).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World Malaria Report. 2018. [Google Scholar]
- 2. White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet 2014; 383:723–35. [DOI] [PubMed] [Google Scholar]
- 3. Miller LH, Ackerman HC, Su XZ, Wellems TE. Malaria biology and disease pathogenesis: insights for new treatments. Nat Med 2013; 19:156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeo TW, Lampah DA, Gitawati R, et al. . Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med 2007; 204:2693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeo TW, Lampah DA, Gitawati R, et al. . Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A 2008; 105:17097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanson J, Lee SJ, Hossain MA, et al. . Microvascular obstruction and endothelial activation are independently associated with the clinical manifestations of severe falciparum malaria in adults: an observational study. BMC Med 2015; 13:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, Anstey NM. Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis 2013; 207:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol 2015; 80:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pillinger NL, Kam P. Endothelial glycocalyx: basic science and clinical implications. Anaesth Intensive Care 2017; 45:295–307. [DOI] [PubMed] [Google Scholar]
- 10. Lopez-Quintero SV, Amaya R, Pahakis M, Tarbell JM. The endothelial glycocalyx mediates shear-induced changes in hydraulic conductivity. Am J Physiol Heart Circ Physiol 2009; 296:H1451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang TH, Alonso S, Ng LF, et al. . Increased serum hyaluronic acid and heparan sulfate in dengue fever: association with plasma leakage and disease severity. Sci Rep 2017; 7:46191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmidt EP, Overdier KH, Sun X, et al. . Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med 2016; 194:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCormick CJ, Craig A, Roberts D, Newbold CI, Berendt AR. Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J Clin Invest 1997; 100:2521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hempel C, Wang CW, Kurtzhals JAL, Staalsø T. Binding of Plasmodium falciparum to CD36 can be shielded by the glycocalyx. Malar J 2017; 16:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hempel C, Hyttel P, Kurtzhals JA. Endothelial glycocalyx on brain endothelial cells is lost in experimental cerebral malaria. J Cereb Blood Flow Metab 2014; 34:1107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hempel C, Sporring J, Kurtzhals JAL. Experimental cerebral malaria is associated with profound loss of both glycan and protein components of the endothelial glycocalyx. FASEB J 2019; 33:2058–71. [DOI] [PubMed] [Google Scholar]
- 17. Yeo TW, Lampah DA, Tjitra E, et al. . Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis 2009; 200:1522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Embery G, Milner AC, Waddington RJ, Hall RC, Langley MS, Milan AM. Identification of proteinaceous material in the bone of the dinosaur Iguanodon. Connect Tissue Res 2003; 44(Suppl 1):41–6. [PubMed] [Google Scholar]
- 19. Sun X, Li L, Overdier KH, et al. . Analysis of total human urinary glycosaminoglycan disaccharides by liquid chromatography-tandem mass spectrometry. Anal Chem 2015; 87:6220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H, Wood T, Young SP, Millington DS. A straightforward, quantitative ultra-performance liquid chromatography-tandem mass spectrometric method for heparan sulfate, dermatan sulfate and chondroitin sulfate in urine: an improved clinical screening test for the mucopolysaccharidoses. Mol Genet Metab 2015; 114:123–8. [DOI] [PubMed] [Google Scholar]
- 21. Husdan H, Rapoport A. Estimation of creatinine by the Jaffe reaction. A comparison of three methods. Clin Chem 1968; 14:222–38. [PubMed] [Google Scholar]
- 22. Kim HY. Statistical notes for clinical researchers: post-hoc multiple comparisons. Restor Dent Endod 2015; 40:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007; 454:345–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med 2016; 280:97–113. [DOI] [PubMed] [Google Scholar]
- 25. Plewes K, Kingston HWF, Ghose A, et al. . Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect Dis 2017; 17:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Finney CA, Hawkes CA, Kain DC, et al. . S1P is associated with protection in human and experimental cerebral malaria. Mol Med 2011; 17:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Punsawad C, Viriyavejakul P. Reduction in serum sphingosine 1-phosphate concentration in malaria. PLoS One 2017; 12:e0180631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lovegrove FE, Tangpukdee N, Opoka RO, et al. . Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 2009; 4:e4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lukasz A, Hillgruber C, Oberleithner H, et al. . Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc Res 2017; 113:671–80. [DOI] [PubMed] [Google Scholar]
- 30. Yeo TW, Lampah DA, Kenangalem E, et al. . Impaired systemic tetrahydrobiopterin bioavailability and increased dihydrobiopterin in adult falciparum malaria: association with disease severity, impaired microvascular function and increased endothelial activation. PLoS Pathog 2015; 11:e1004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubach MP, Mukemba J, Florence S, et al. . Impaired systemic tetrahydrobiopterin bioavailability and increased oxidized biopterins in pediatric falciparum malaria: association with disease severity. PLoS Pathog 2015; 11:e1004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lopansri BK, Anstey NM, Weinberg JB, et al. . Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet 2003; 361:676–8. [DOI] [PubMed] [Google Scholar]
- 33. Anstey NM, Weinberg JB, Hassanali MY, et al. . Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med 1996; 184:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yeo TW, Lampah DA, Tjitra E, et al. . Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog 2010; 6:e1000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weinberg JB, Yeo TW, Mukemba JP, et al. . Dimethylarginines: endogenous inhibitors of nitric oxide synthesis in children with falciparum malaria. J Infect Dis 2014; 210:913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weinberg JB, Volkheimer AD, Rubach MP, et al. . Monocyte polarization in children with falciparum malaria: relationship to nitric oxide insufficiency and disease severity. Sci Rep 2016; 6:29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manjurano A, Sepúlveda N, Nadjm B, et al. ; in Collaboration With MalariaGEN USP38, FREM3, SDC1, DDC, and LOC727982 gene polymorphisms and differential susceptibility to severe malaria in Tanzania. J Infect Dis 2015; 212:1129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tarbell JM, Simon SI, Curry FR. Mechanosensing at the vascular interface. Annu Rev Biomed Eng 2014; 16:505–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colbert JF, Schmidt EP. Endothelial and microcirculatory function and dysfunction in sepsis. Clin Chest Med 2016; 37:263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.