Figure 1.
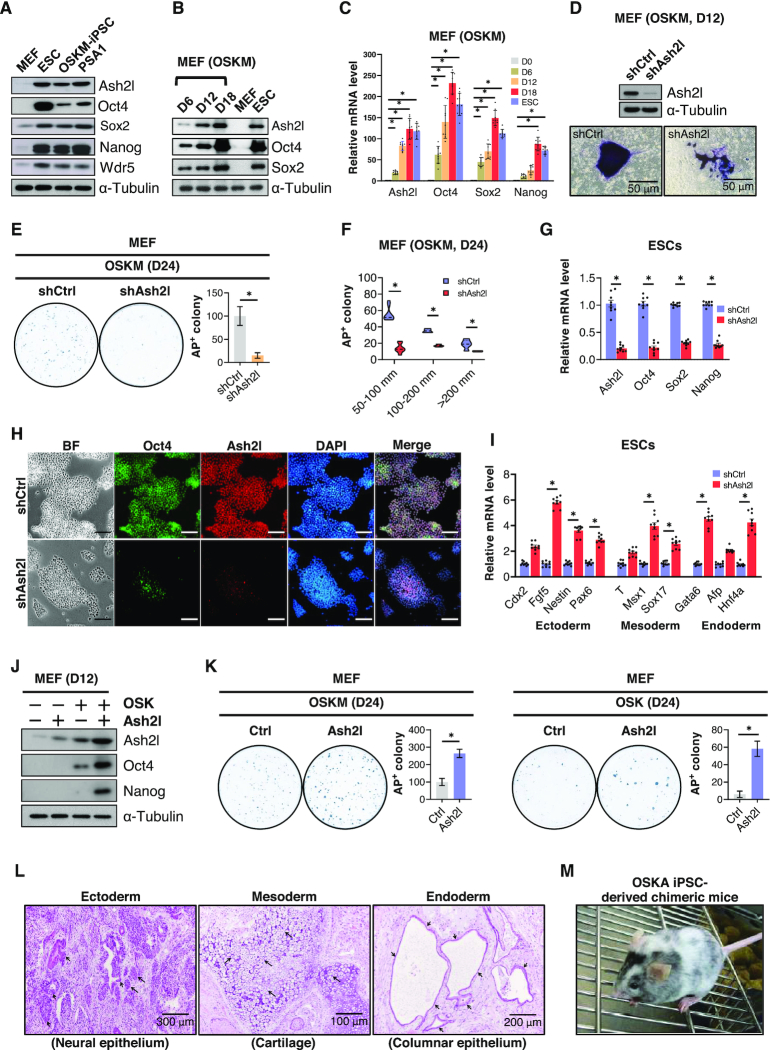
Ash2l expression is essential for iPSC generation. (A) Western blot shows the protein expression of Ash2l, Oct4, Sox2, Nanog and Wdr5 in mouse fibroblasts (MEFs), mouse embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) and embryonal carcinoma cell line PSA1. α-Tubulin was used as loading control. (B) Time-course western blot shows the changes in the protein content of Ash2l, Oct4, Sox2, Wdr5 and Klf4 during the reprogramming process of MEFs. The reprogramming was induced by infecting conventional reprogramming factors Oct4, Sox2, Klf4 and c-Myc (OSKM) in MEFs. Non-infected MEFs and ESCs were used as negative and positive controls, respectively. α-Tubulin was used as loading control. (C) A time-course qPCR shows the changes in the mRNA expression of Ash2l, Oct4, Sox2, Nanog, Nr5a2 and Wdr5 during reprogramming. (D) Alkaline phosphatase (AP) staining shows the reprogramming colony size of control or Ash2l knockdown MEFs at day 12 post-reprogramming. Control (shCtrl) and Ash2l knockdown (shAsh2l) MEFs were subjected to the reprogramming process using OSKM factors. Twelve days after reprogramming, the efficiency of Ash2l knockdown was confirmed by western blot (upper), and MEFs were then subjected to AP staining (lower). (E) Reprogramming efficiency of MEFs with or without Ash2l knockdown. MEFs were reprogrammed by OSKM with or without Ash2l knockdown. Reprogrammed MEFs were stained with AP 24 days after reprogramming (left), and the numbers of AP-positive colonies (right) in each plate were calculated. (F) Measurements of the colony numbers of OSKM-reprogrammed MEFs with or without Ash2l knockdown at all given colony size at day 24 post-reprogramming. Data are presented as mean ± SD; *P< 0.01. (G) qPCR analysis of pluripotency genes (Oct4, Sox2 and Nanog) in ESCs with or without Ash2l knockdown. (H) Immunofluorescence staining showing the Oct4 protein expression in ESCs with or without Ash2l-knockdown. DAPI was used for staining the nucleus; scale bar = 100 μm. (I) qPCR analysis of development-related genes (ectoderm: Cdx2, Fgf5, Nestin, Pax6; mesoderm endoderm: T, Msx1, Sox17; endoderm: Gata6, Afp, HNF4α) in ESCs with or without Ash2l knockdown. (J) Western blot shows the expression of endogenous Ash2l, Oct4 and Nanog protein in OSK-reprogrammed MEFs with or without Ash2l overexpression. α-Tubulin was used as loading control. (K) Reprogramming efficiency of MEFs infected with OSKM (left) or OSK (right), with or without Ash2l overexpression. Reprogrammed MEFs were subjected to AP staining 24 days after infection, and the numbers of AP-positive colonies in each plate were calculated. Data are presented as mean ± SD; *P< 0.01. (L) Ex vivo biopsies and histological analysis reveal the teratoma formation in the grafts of OSK- and Ash2l-reprogrammed iPSCs in the dorsal flanks of nude mice. Sections of the teratoma tissues were subjected to hematoxylin and eosin staining for the examination of the formation of three germ layers. Ectoderm: neuronal epithelium; Mesoderm: cartilage and keratinocytes; Endoderm: columnar epithelium. (M) Competence of OSK/Ash2l-reprogrammed iPSCs to generate chimeric mice is confirmed by coat color. Western blot in panels (A), (B), (D) and (J) are representatives of three separate experiments using independent cell preparations. The quantification of alkaline phosphatase staining shown in panels (E) and (K) are the mean ± SD of six independent experiments. Data are normalized to Gapdh and are presented as mean ± SD; *P< 0.01; see also Supplementary Figure S1.