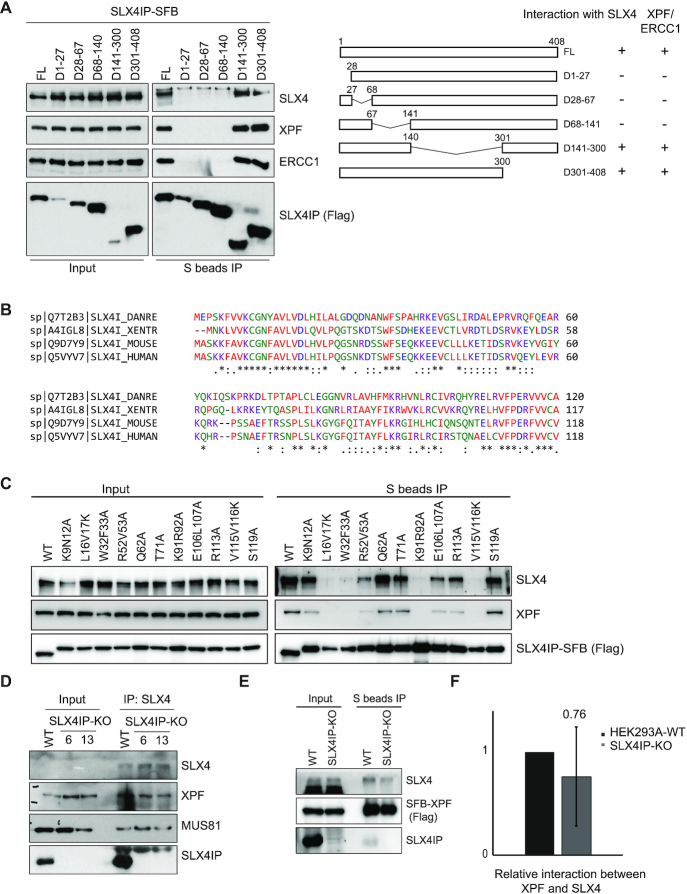
Figure 4.
The conserved N-terminus of SLX4IP is responsible for its interaction with SLX4–XPF–ERCC1. (A) HEK293A cells were transfected with constructs encoding SLX4IP-SFB or its deletion variants and subjected to immunoprecipitation (IP) pulldown with S beads. Western blotting conducted with antibodies as indicated. A schematic overview of the deletion strategy and protein-protein interaction results is presented. (B) Conservation of sequences at the N-terminus of SLX4IP. The alignment of the N-terminal sequences of SLX4IP in humans, mice, zebrafish and Xenopus, established using the Clustal Omega sequence alignment program, is shown. (C) HEK293A cells were transfected with constructs encoding SLX4IP-SFB or its point mutation variants and subjected to IP with S beads. Western blotting was conducted with antibodies as indicated. (D) SLX4 was subjected to IP with anti-SLX4 serum from HEK293A-WT or SLX4IP-KO cells (clone #6 and clone #13). Western blotting was conducted with antibodies as indicated. (E) HEK293A-WT or SLX4IP-KO cells were transfected with SFB-XPF and subjected to IP with S beads. Western blotting was conducted with antibodies as indicated. (F) Western blots detecting the interaction between XPF and SLX4 in HEK293A-WT and SLX4IP-KO cells were analyzed by ImageJ. The mean relative interaction from five experiments is shown. FL, full length (controls); KO, knockout; WT, wild type.