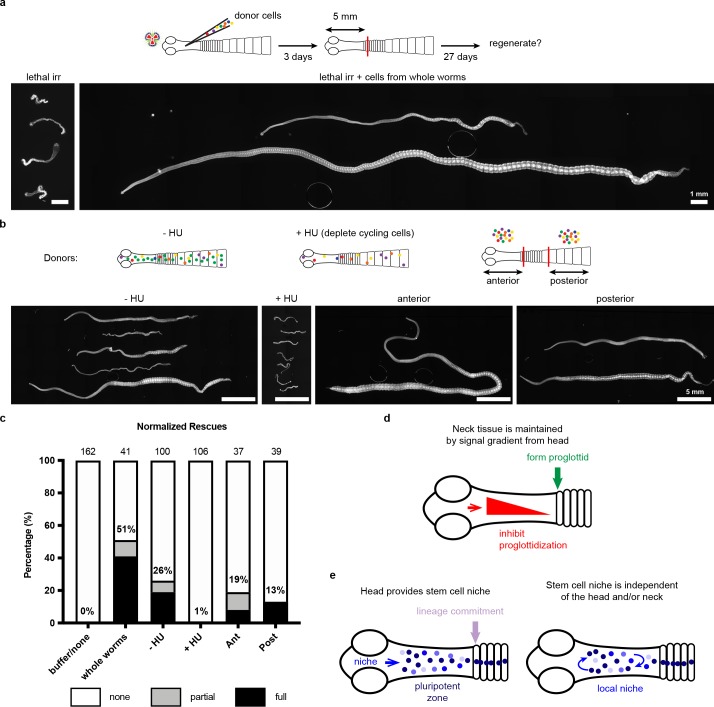
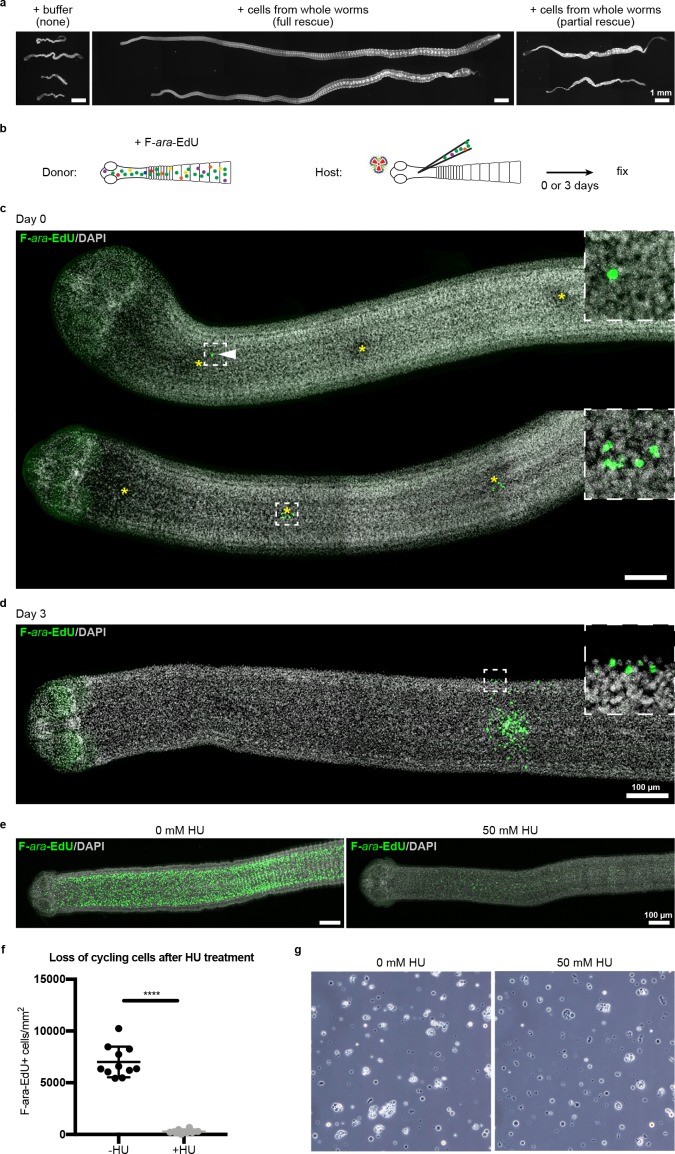
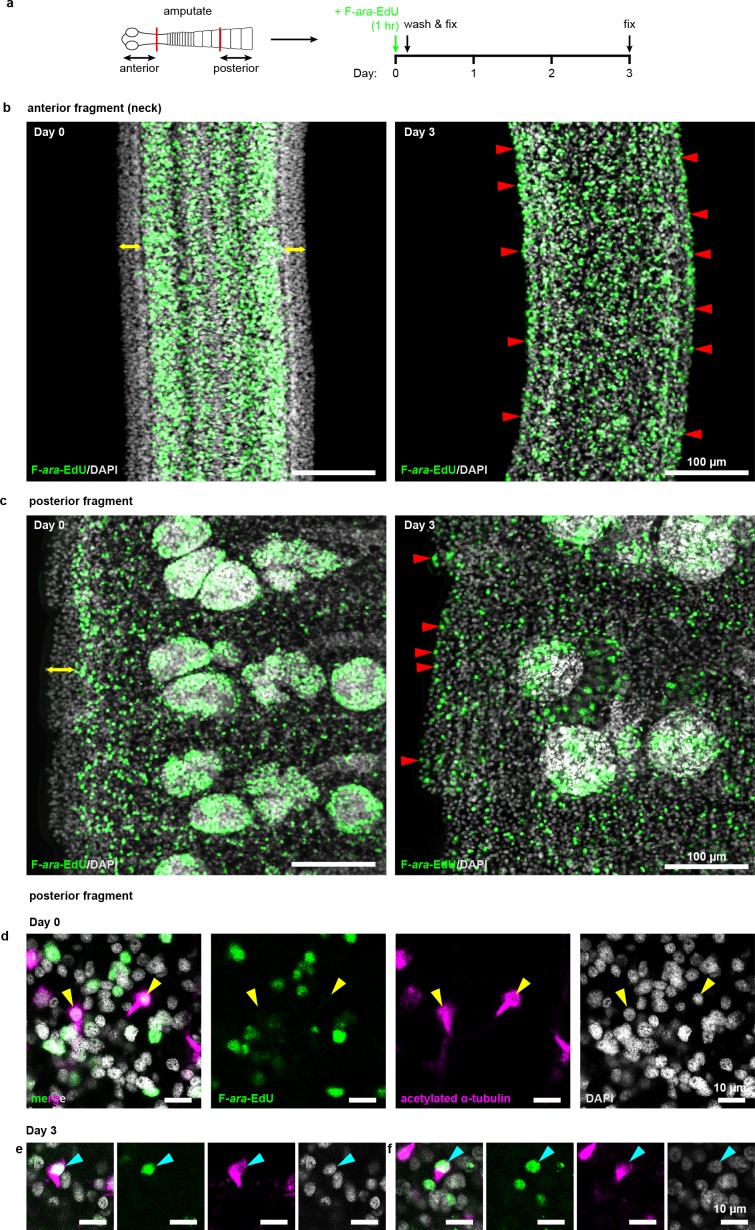
(a) DAPI-stained worms showing phenotypes observed after attempted rescue of irradiation-induced lethality. No rescue results in degenerated worms with no proglottids, full rescue results in normal worms with multiple proglottids, and partial rescue refers to worms with visible proglottids but with defects such as contracted necks. (b) Schematic for rescue experiment using donors with labeled cycling cells. (c–d) Maximum-intensity projections of tile-stitched confocal sections 0 or 3 days post-transplantation according to (b). Injections sites marked with asterisks. White arrowhead points to a single transplanted cell. After 3 days, large colonies of F-ara-EdU+ (green) cells could be detected with some labeled cells incorporated into terminally differentiated tissues at the animal edge (inset). (e) Maximum-intensity projections of tile-stitched confocal sections after 1 hr F-ara-EdU uptake (green) from control worms or worms cultured with hydroxyurea (HU) for 6 days. (f) Quantification of cycling cells from (e). Error bars = SD, N = 3, n = 11 and 8, Student’s t-test. (g) Cell morphology with or without HU treatment prior to transplantation.