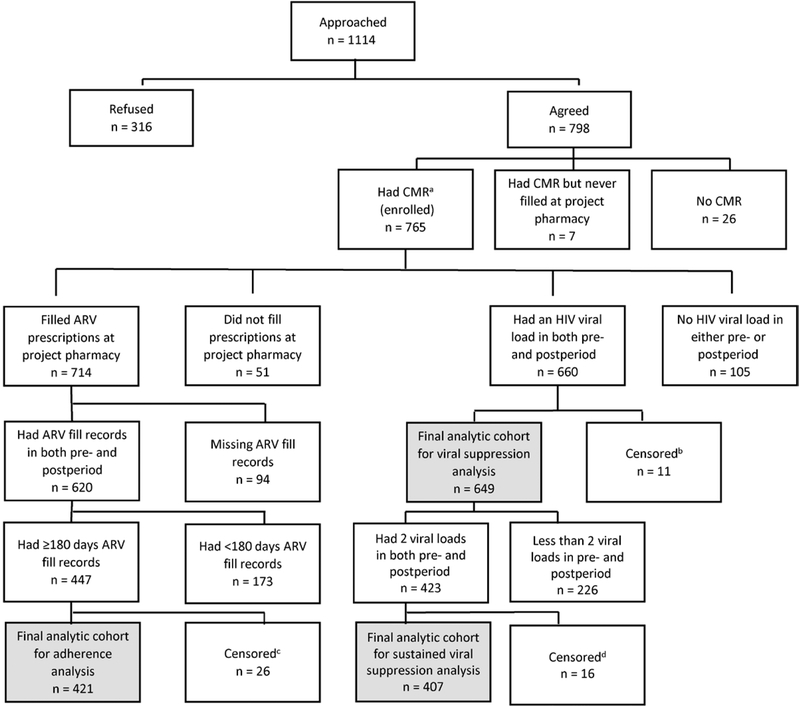
Figure 1.
Flow diagram of inclusion in adherence and viral suppression analyses, Patient-centered Human Immunodeficiency Virus (HIV) Care Model, 2014–2016, United States. Abbreviations: ARV, antiretroviral; CMR, comprehensive medication review; HIV, human immunodeficiency virus. aAll persons who completed an initial comprehensive medication review and who filled prescriptions at the project pharmacy were considered enrolled in the project. bPersons were censored in the postimplementation period before a viral load test was drawn and were excluded from the viral suppression analysis. Persons were censored for the following reasons: 1 voluntarily withdrew, 1 was incarcerated, 1 moved out of area, and 8 transferred prescriptions to a nonproject (or nonproject network) pharmacy. cPersons were censored in the postimplementation period and excluded from the adherence analysis. Persons were censored for the following reasons: 5 died, 2 moved out of area, 2 transferred care, 1 was incarcerated, 2 voluntarily withdrew, and 9 transferred prescriptions to a nonproject (or nonproject network) pharmacy. Two project sites did not collect censoring data. For individuals from these 2 sites, persons were censored 1 day after the date of the last clinic visit if a person had no clinic visit or HIV laboratory test drawn for >6 months but continued to fill prescriptions in the last 6 months of the project implementation period; 5 persons were censored for this reason. dPersons were censored in the postimplementation period before 2 viral load tests were drawn and were excluded from the sustained viral suppression analysis. Persons were censored for the following reasons: 1 died, 1 transferred care, 1 moved out of area, 1 was incarcerated, 2 transferred prescriptions to a nonproject (or nonproject network) pharmacy, and 9 were no longer able to fill prescriptions at project pharmacy due to insurance reasons. Two project sites did not collect censoring data. For individuals from these 2 sites, persons were censored 1 day after the date of the last clinic visit if a person had no clinic visit or HIV laboratory test drawn for >6 months but continued to fill prescriptions in the last 6 months of the project implementation period; 1 person was censored for this reason.