Extensive xanthelasma-like lesions of the eyelids is the most frequent cutaneous manifestation of Erdheim-Chester disease (ECD).1 Recently, mutations activating the MAPK pathway have been found in ECD patients and it has been suggested in the revised classification of histiocytic disorders that all extracutaneous or disseminated juvenile xanthogranulomata with gain-of-function mutation of BRAF, NRAS, KRAS, or MAP2K1, should be considered as ECD.2 Chronic myelomonocytic leukemia (CMML) is a rare clonal hematopoietic disorder that presents features of both myeloproliferative neoplasm and myelodysplastic syndrome. Its diagnosis requires a persistent peripheral blood monocytosis >1x109/L after exclusion of a reactive monocytosis; myelodysplastic features and/or the presence of an acquired clonal cytogenetic or a mutational abnormality in hematopoietic cells.3 Specific skin lesions in CMML are rare with an estimated prevalence of 10%.4 Diffuse or periorbital xanthomatous lesions associated with CMML have been reported.5,6 Some authors suggested that xanthomatous lesions may be specific cutaneous involvement of CMML,7 however this has never been confirmed. Furthermore, an association between CMML and ECD has been reported8 and the recent classification of histiocytoses added a subtype of ECD associated with another myeloproliferative disorder.2 Mutations activating the MAPK pathway including KRAS, NRAS and BRAF have also been described in CMML patients.9 However, the clonal relationship between the two diseases has not been clearly demonstrated.8
We report herein three cases of xanthelasma-like lesions fulfilling clinical, histological and molecular criteria for ECD associated with CMML with a proven clonal relationship between the skin lesions and CMML cells based on molecular analyses.
Briefly, for next-generation targeted sequencing (NGS) of CMML cells, genomic DNA was extracted from the bone marrow (patient 1 and 3) or from peripheral blood mononuclear cells (patient 2). A panel of 41 genes frequently mutated in myeloid malignancies was designed including ASXL1, ASXL2, ATM, BCOR, CALR, CBL, CEBPA, CSF3R, DDX41, DNMT3A, EZH2, FLT3, GATA2, HRAS, IDH1, IDH2, IKZF1, JAK2, KIT, KMT2A, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SH2B3, SMC1A, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, WT1 and ZRSR2. Libraries were obtained from 112.5 or 200 ng of DNA according to the manufacturer’s instructions, using HaloPlex Target Enrichment System® (Agilent technologies), and sequenced on a MiSeq® sequencer (IlluminaINC). Read alignment, variant calling, and annotation were performed using Sophia DDM® software version 5.0.12 (Sophia genetics). The sensitivity was 1%. All variants were checked using Intergated Genomics Viewer (IGV) software v2.3.
For formalin-fixed and paraffin-embedded (FFPE) skin biopsies, NGS was not available routinely and therefore biopsies were sent to the Department of Pathology of Ambroise Pare Hospital. Genomic DNA was extracted from FFPE fragments as previously described.10 A custom panel of 76 genes frequently mutated in cancers (hot spots) and in myeloid malignancies was used including AKT1, ALK, ARAF, ASXL1, BRAF, CALR, CBL, CDK4, CDKN1B, CDKN2A, CEBPA, CSF1R, CSF3R, CTNNB1, DNMT3A, EGFR, ETV6, EZH2, FLT3, GATA2, GNA11, GNAQ, GNAS, HERC1, HRAS, IDH1, IDH2, JAK2, JAK3, KIT, KRAS, KTM2D, MAML3, MAMLD1, MAP2K1, MAP2K2, MAP2K3, MAP2K4, MAP2K6, MAP3K1, MAP3K8, MAP3K9, MAP3K10, MAP3K19, MAP4K4, MAPK1, MAPK11, MAPK9, MPL, NF1, NOTCH1, NOTCH2, NPM1, NRAS, PDGFRA, PIK3CA, PPP6C, PTEN, PTPN11, RAC1, RAF1, RIT1, RUNX1, SETBP1, SF3B1, SRSF2, STAG2, STK19, SYNGAP1, TAOK1, TAOK2, TET2, TP53, U2AF1, WT1, ZRSR2(the 30 mutual genes between both panels are highlighted in bold). DNA quality was evaluated with the Agilent NGS FFPE QC KiT (Agilent) and libraries were prepared from 25-150 ng of DNA depending of its quality using TruSeq®Custom Amplicon Low Input kit (Illumina), and were sequenced on a Miseq sequencer (Illumina). Read alignment, variant calling, and annotation were performed using GensearchNGS v.1.6.31 (PhenoSystems SA). The sensitivity was 2%. All variants were checked using IGV software v2.3. The study was conducted according to the French CNIL methodology reference and in accordance with the Declaration of Helsinki.
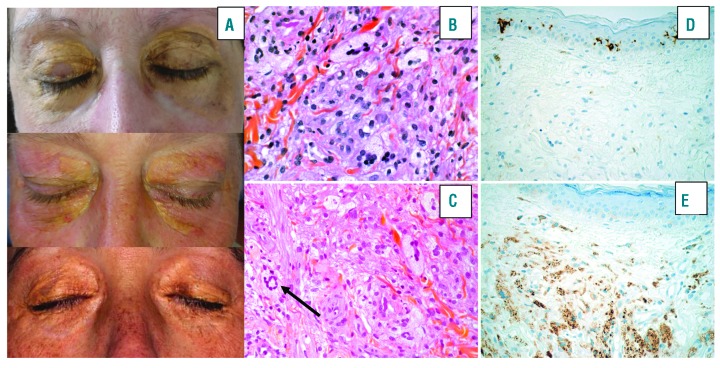
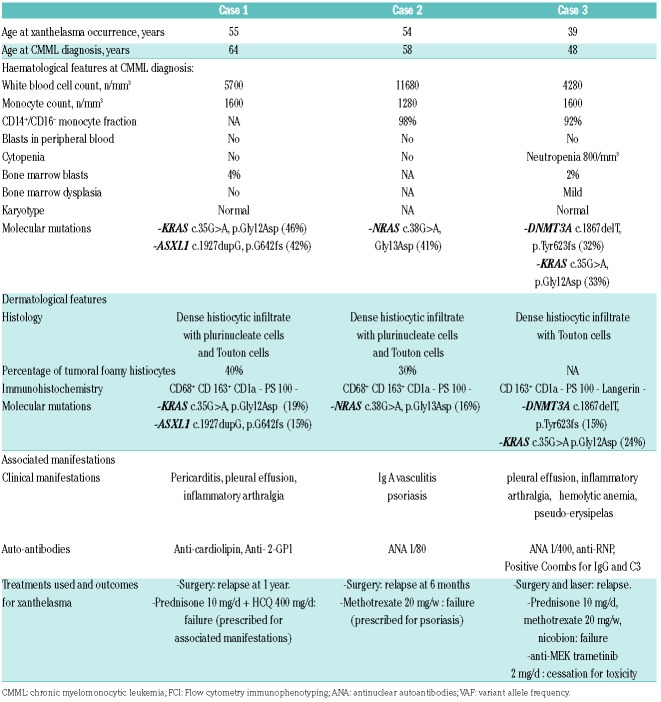
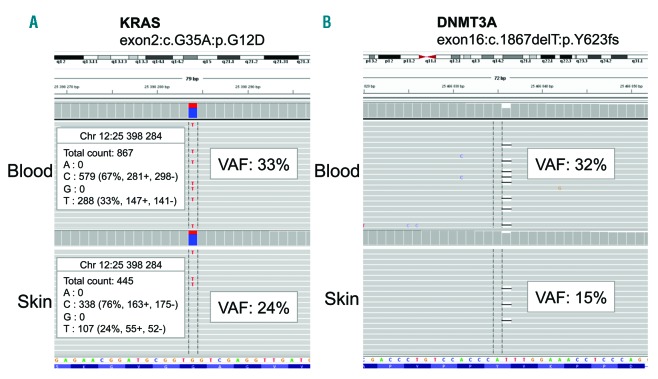
Case reports: three women between 52 and 62 years of age presented with a long-term history of extensive periorbital and circumferential xanthomatous lesions (Figure 1 A). Clinical, hematological, histological and molecular features are presented in Table 1. All of them had a history of CMML-0 based on the results of blood samples, bone marrow (BM) examination and/or flow cytometry immunophenotyping. Circulating monocytes were measured to be between 1.0-1.6×109/L and no specific treatment for CMML was required. NGS studies performed on DNA obtained from the BM aspirate or blood samples identified the following mutations KRAS c.35G>A p.(Gly12Asp) (variant allele frequency (VAF): 0.46) and ASXL1 31022441-chr20c.1926-1927insG (VAF: 0.42) for patient 1; NRAS mutation c38G>A, p.(Gly13Asp) (VAF: 0.41) for patient 2 and KRAS (exon2:c.G35A:p.G12D) (VAF 0.33) and DNMT3A (exon 16:c.1867delT:p.Y623fs) (VAF: 0.32) for patient n°3. Skin biopsies of periorbital lesions of the eyelid revealed a similar pattern with a dense mononuclear infiltrate of the dermis, extending to the dermo-hypodermic junction. The infiltrate consisted of macrophages with foamy cytoplasm and a small central nucleus, and some multinucleated cells and Touton cells. Immunohistochemical studies showed that these histiocytes were negative for CD1a and S100, positive for CD163 and CD68 (Figure 1B-E). These findings were similar to those observed in ECD.1 Given the history of CMML together with histological findings suggestive of ECD, NGS analyses were also performed on DNA obtained from FFPE tissue sections of the eyelids to investigate the relationship between the histiocytes comprising the skin lesions and the CMML clones. These studies identified the same mutations as those found in the CMML cells from the BM aspirate or blood samples (Table 1 and Figure 2). Positron emission tomography and computed tomography did not show bone lesions, perinephric fat or periaortic infiltration suggestive of systemic involvement of ECD. However, these cases were classified as ECD based on the identification of a clonal mutation of the MAPK pathway in cutaneous lesions with clinical and pathological findings of adult-xanthogranuloma as suggested by the revised classification of histiocytoses.2
Figure 1.
Clinicopathological features of cases. A. Clinical features of xanthomatous lesions of the eyelids. Extensive, multiple yellowish papules/plaques with color variations from yellowish to gold located on the inner and outer canthus of the eyelids with extension to the cheek and temporal area. B, C, D, E. Biopsies of the eyelids. (B, C) Dense dermal infiltrate comprised of macrophages with foamy cytoplasm and small central nucleus, some multinucelated cells and Touton cells (black arrow). (D) Immunostaining for CD1a was negative and diffusely positive for CD68 (E). (B and C, Hematoxylineosin stain; D and E, Immunoperoxydase stain; original magnification 400x).
Table 1.
Clinical, biological, histological and molecular features of included patient with extensive xanthomatous lesions of the eyelid associated with CMML.
Figure 2.
Next generation sequencing data from patient number 3 on DNA obtained from a peripheral blood sample (up) and the eyelid skin lesion (down), showing the same two identical mutations in both samples. (A) KRAS c.35G>A, p.Gly12Asp mutation, (B) DNMT3A c.1867delT, p.Tyr623fs mutation. Data are shown as screenshots from the Integrated Genomics Viewer visualization tool (http://software.broadinstitute.org/software/igv). For base substitution, the read counts for each base are indicated. VAF, variant allelic frequency.
We report three cases of extensive xanthelasma-like lesions of the eyelids clinically and histologically similar to those observed in adult-xanthogranuloma or ECD1 fulfilling criteria for both ECD and CMML.2,3 We identified the same mutations in the skin ECD lesions and the CMML cells, demonstrating a clonal relationship between both diseases. Of note, although all patients had monocytosis at the time of the skin biopsy, a contamination by blood CMML monocytes is unlikely. Indeed, the VAF ranged from 15 to 24%, corresponding to 30 to 48% of cells with a heterozygous mutation consistent with the percentage of tumoral histiocytes in skin biopsies (Table 1). Moreover, the three patients had mild monocytosis, and only <5% monocytes were present within areas of the dermis selected for molecular analysis.
Recently, Papo et al. identified that 19/189 of ECD were associated with myeloid neoplasms including eight CMML. In the only patient who had NGS targeted sequencing in both ECD and CMML cells, the same NRAS mutations were identified in perirenal tissue suggesting that the association is not restricted to ECD with limited skin involvement as in our cases.8 Moreover, recently, Ghobadi et al. identified the same BRAF-V600E mutation in the blasts from BM aspirate of an acute myeloid leukemia (AML) M5 and in ECD cells from a lung biopsy with bone, periaortic infiltration, lung, neurological and retroperitoneal involvement.11 Moreover, using whole exome sequencing, they confirmed that the ECD and AML had multiple shared mutations and arose from the same cell of origin with additional mutations mostly for AML cells.11 Some authors suggested that cutaneous involvement of CMML may be clonal.12 Using fluorescence in situ hybridization, Vitte et al. found the same karyotype in cutaneous lesions and blood of 4/8 CMML patients.12
Additionally, two recent studies highlighted that the cell of origin of one third of patients with systemic histiocytosis resides in CD34+ hematopoietic progenitor cells prior to committed monocytes/macrophages.13,14 Two main biologic mechanisms might explain the link between CMML and ECD. The first one would be a differentiation from a clonal CMML cell into foamy macrophages in the skin through an additional genetic hit or a transforming event. The second would be a mutant common progenitor cell giving rise to the two distinct neoplasms via divergent differentiation. We identified the same mutations in CMML cells and skin lesions without additional mutations, which may favor the first hypothesis. Moreover, Milne et al. showed that monocytes could differentiate into foamy macrophages in vitro with macrophage colony-stimulating factor and human serum.14 However, contrary to the case of Ghobadi et al.11 exome sequencing has not been performed in our cases and therefore, further studies are needed to confirm this hypothesis.
Overall, the high prevalence of myeloid neoplasms including CMML in adults with non-Langerhans cell histiocytosis8 as well as in patients with Langerhans cell histiocytosis may be explained by a common bone marrow precursor.13,15 We report herein three cases of disseminated xanthelasma-like lesions of the eyelids fulfilling criteria for ECD2 associated with CMML harboring the same mutations in skin biopsies and CMML cells. This study provides further evidence for a common progenitor cell between CMML and ECD in some cases.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Chasset F, Barete S, Charlotte F, et al. Cutaneous manifestations of Erdheim-Chester disease (ECD): Clinical, pathological, and molecular features in a monocentric series of 40 patients. J Am Acad Dermatol. 2016;74(3):513–520. [DOI] [PubMed] [Google Scholar]
- 2.Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. [DOI] [PubMed] [Google Scholar]
- 4.Mathew RA, Bennett JM, Liu JJ, et al. Cutaneous manifestations in CMML: Indication of disease acceleration or transformation to AML and review of the literature. Leuk Res. 2012;36(1):72–80. [DOI] [PubMed] [Google Scholar]
- 5.Oka M, Okamura A, Kawano S, Fukumoto T, Sakaguchi M, Nishigori C. Diffuse plane normolipemic xanthoma associated with chronic myelomonocytic leukemia-1. Eur J Dermatol. 2014; 24(1):112–113. [DOI] [PubMed] [Google Scholar]
- 6.Edwards LR, Kidd LL, Gru AA, Wilson BB. Image Gallery: Necrobiotic xanthogranuloma in association with chronic myelomonocytic leukaemia. Br J Dermatol. 2017;176(2):e16. [DOI] [PubMed] [Google Scholar]
- 7.Miralles ES, Escribano L, Bellas C, Núñez M, Ledo A. Cutaneous xanthomatous tumours as an expression of chronic myelomonocytic leukaemia¿ Clin Exp Dermatol. 1996;21(2):145–147. [PubMed] [Google Scholar]
- 8.Papo M, Diamond EL, Cohen-Aubart F, et al. High prevalence of myeloid neoplasms in adults with non-Langerhans cell histiocytosis. Blood. 2017;130(8):1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Singh RR, Patel KP, et al. BRAF kinase domain mutations are present in a subset of chronic myelomonocytic leukemia with wild-type RAS. Am J Hematol. 2014;89(5):499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colomba E, Hélias-Rodzewicz Z, Von Deimling A, et al. Detection of BRAF p.V600E mutations in melanomas: comparison of four methods argues for sequential use of immunohistochemistry and pyrosequencing. J Mol Diagn. 2013;15(1):94–100. [DOI] [PubMed] [Google Scholar]
- 11.Ghobadi A, Miller CA, Li T, et al. Shared cell of origin in a patient with Erdheim Chester disease and acute myeloid leukemia. Haematologica. 2019. March 28 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitte F, Fabiani B, Bénet C, et al. Specific skin lesions in chronic myelomonocytic leukemia: a spectrum of myelomonocytic and dendritic cell proliferations: a study of 42 cases. Am J Surg Pathol. 2012;36(9):1302–1316. [DOI] [PubMed] [Google Scholar]
- 13.Durham BH, Roos-Weil D, Baillou C, et al. Functional evidence for derivation of systemic histiocytic neoplasms from hematopoietic stem/progenitor cells. Blood. 2017;130(2):176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milne P, Bigley V, Bacon CM, et al. Hematopoietic origin of Langerhans cell histiocytosis and Erdheim-Chester disease in adults. Blood. 2017;130(2):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiavash K, Malone JC. Langerhans cell histiocytosis associated with underlying hematolymphoid disorders in adults: report of 2 cases and review of the literature. Am J Dermatopathol. 2018;40(8):588–593. [DOI] [PubMed] [Google Scholar]