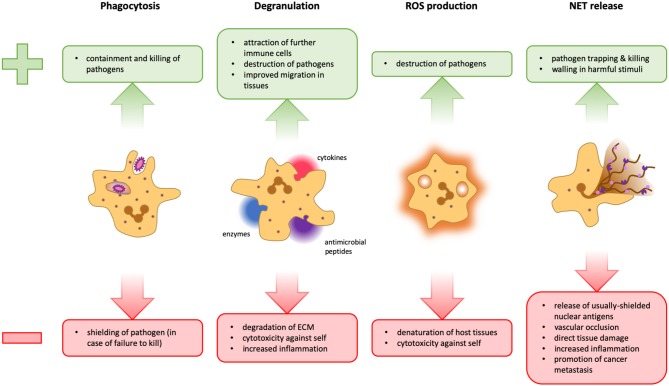
Figure 1.
Advantages and disadvantages of neutrophil effector functions. During phagocytosis, microbes are engulfed and degraded in specialized organelles called phagolysosomes. This is a very powerful and clean method to dispose of pathogens because it takes place in a contained space, thus preventing widespread inflammation. However, should a pathogen manage to survive intracellular degradation, it is protected from extracellular factors and other immune cells that could potentially contain it (Left). Neutrophils can release several different cytokines, antimicrobial peptides, and granular enzymes into their surroundings, a response termed degranulation. This mechanism facilitates migration within the tissue, activates and attracts other immune cells and can help fight pathogens that cannot be phagocytosed. However, the release of too many cytokines can lead to an overshooting immune activation. Tissue degradation could aid the spreading of pathogens and destroy the structural basis of organs, and several granular proteins are also toxic for host cells (Middle Left). Neutrophils can release reactive oxygen species (ROS) into phagolysosomes as well as into the extracellular space. These molecules can potentiate pathogen killing, but are not selective and can therefore also damage host cells (Middle Right). The formation of NETs can be an efficient means to trap and kill pathogens and potentially walling off harmful stimuli. However, the release of cytoplasmic and nuclear proteins can cause the formation of autoantibodies, favoring autoimmunity. Furthermore, NETs can obstruct blood vessels and glandular ducts leading to inflammation. Granular proteins attached to the chromatin fibrils damage host tissue and the release of pro-inflammatory mediators may result in overshooting inflammation. Moreover, NETs have also been implicated in the facilitation of tumor metastasis (Right).