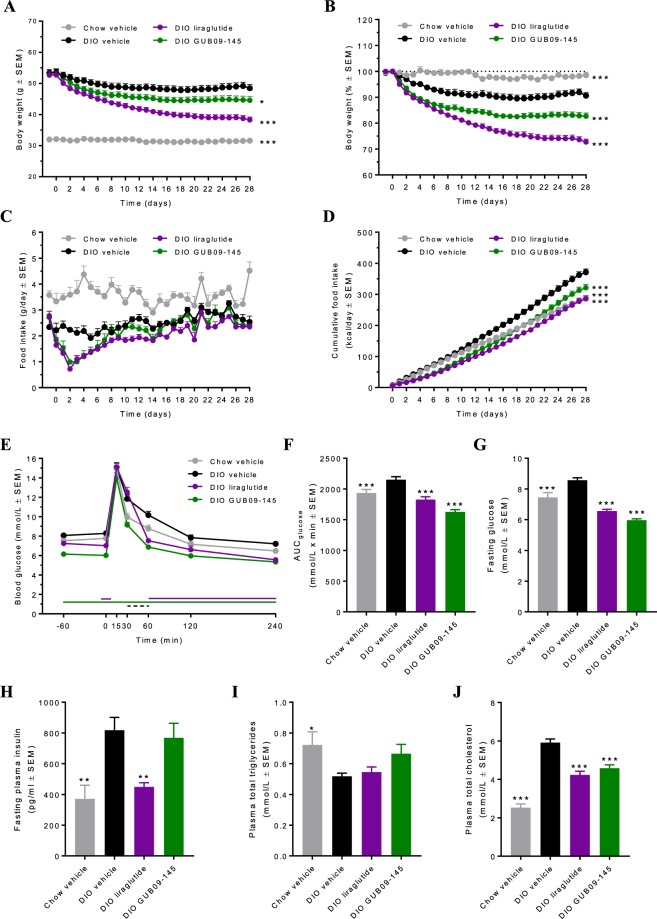
Figure 1.
Liraglutide and GUB09-145 improve metabolic parameters in DIO mice. (A) Absolute body weight (g), (B) Body weight gain (%) relative to treatment start; (C) Daily food intake (g); (D) Cumulative energy intake (kcal/day); (E) Oral glucose tolerance test (OGTT) on treatment day 27, (F) Glucose area-under the-curve (glucose AUC0-240 min); (G) Fasting blood glucose concentrations (mmol/L) on treatment day 14; (H) Fasting plasma insulin levels (pg/ml) on treatment day 28; (I) Plasma total triglycerides (TG, mmol/L) on treatment day 28; (J) Plasma total cholesterol (TC, mmol/L) on treatment day 28. *p < 0.05, **p < 0.01, ***p < 0.001 compared to DIO vehicle controls.