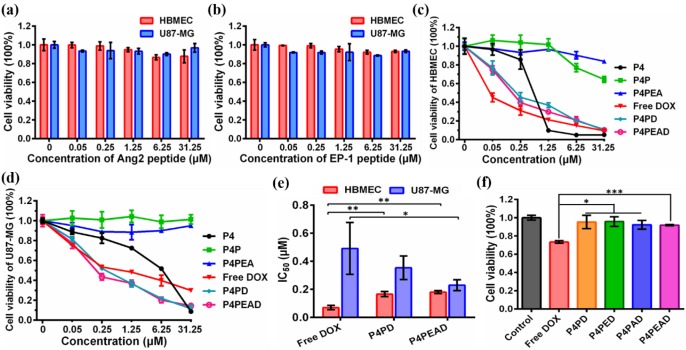
Figure 4.
In vitro evaluation of biocompatibility and antitumor efficacy of the dual-targeting drug delivery system. (a, b) Cytotoxicity of peptides (a) Ang2 and (b) EP-1 to HBMEC and U87-MG cells. (c, d) Cytotoxicity of blank dendrimer-based carriers (P4, P4P and P4PEA), free DOX, P4PD and P4PEAD to (c) HBMEC and (d) U87-MG cells. The cells viability was checked by MTS assay after incubating the cells with different concentrations of peptides, blank carriers and DOX-loaded dendrimer carriers for 48 h. Error bars represent standard deviation (n = 5). (e) IC50 values of different DOX-loaded dendrimers fitted by Origin 8.1 software. Error bars represent standard deviation (n = 5). (f) Short-term cytotoxicity of different DOX formulations to HBMEC cells after incubating the cells with the DOX formulations for 3 h at the DOX concentration of 20 μΜ. Error bars represent standard deviation (n=5). *p < 0.1, **p < 0.01, ***p < 0.001 (Student's t-test).