SUMMARY
SETTING:
Sixty-seven government health facilities providing tuberculosis (TB) and human immunodeficiency virus (HIV) services across Ethiopia.
OBJECTIVE:
To examine clinician barriers to implementing isoniazid preventive therapy (IPT) among people living with HIV.
DESIGN:
A cross-sectional study to evaluate the provider-related factors associated with high IPT coverage at the facility level.
RESULTS:
On bivariate analysis, the odds of high IPT implementation were lower when clinicians felt patients were negatively affected by the side effects of IPT (OR 0.18, 95%CI 0.04–0.81) and perceived that IPT increased multidrug-resistant TB (MDR-TB) rates (OR 0.66,95%CI 0.44–0.98). The presence of IPT guidelines on site (OR 2.93, 95%CI 1.10–7.77) and TB-HIV training (OR 3.08, 95%CI 1.11–8.53) had a positive relationship with high IPT uptake. In the multivariate model, clinician’s perception that active TB was difficult to rule out had a negative association with a high IPT rate (OR 0.93; 95%CI 0.90–0.95).
CONCLUSIONS:
Clinician impression that ruling out active TB among HIV patients is difficult was found to be a significant barrier to IPT uptake. Continued advancement of IPT relies greatly on improving the ability of providers to determine IPT eligibility and more confidently care for patients on IPT. Improved clinician support and training as well as development of new TB diagnostic technologies could impact IPT utilization among providers.
Keywords: isoniazid preventive therapy, latent tuberculous infection, TB-HIV, Ethiopia
Abstract
RÉsumÉ
CONTEXTE :
Soixante-sept structures de santé publiques offrant des services de tuberculose (TB) et de virus de l’immunodéficience humaine (VIH) à travers l’Ethiopie.
OBJECTIF :
Examiner les obstacles de la part des cliniciens à la mise en œuvre du traitement préventif par l’isoniazide (IPT) parmi les personnes vivant avec VIH.
SCHÉMA :
Etude transversale visant à évaluer les facteurs liés aux prestataires de soins associés à une couverture élevée de l’IPT au niveau des structures de santé.
RÉSULTATS :
Une analyse bivariée a constaté; que les probabilités de mise en œuvre élevée de l’IPT ont été plus faibles quand les cliniciens trouvaient que les patients étaient négativement affectés par les effets secondaires de l’IPT (OR 0,18 ; IC95% 0,04–0,81) et pensaient que l’IPT augmentait les taux de TB multirésistante (OR 0,66 ; IC95% 0,44–0,98). La présence des directives relatives à l’IPT sur le site (OR 2,93 ; IC95% 1,10–7,77) et la formation TB-VIH (OR 3,08 ; IC95% 1,11–8,53) ont eu une relation positive avec une couverture élevée de l’IPT. En modèle multivarié, la perception des cliniciens selon laquelle la TB active a été difficile à éliminer a eu une association négative avec un taux élevé d’IPT (OR 0,93 ; IC95% 0,90–0,95).
CONCLUSIONS :
L’impression des cliniciens selon laquelle il est difficile d’éliminer la TB active parmi les patients VIH s’est avérée être un obstacle significatif à la couverture de l’IPT dans cette étude. La poursuite du progrès de l’IPT repose grandement sur l/amélioration de la capacité des prestataires de soins à déterminer l’éligibilité à l’IPT et à prendre en charge avec plus de confiance les patients sous IPT. Davantage de soutien et de formation aux cliniciens ainsi que l’élaboration de nouvelles techniques de diagnostic de TB ont le potentiel d’avoir un impact positif sur l’utilisation de l’IPT parmi les prestataires de soins.
Abstract
MARCO DE REFERENCIA:
Sesenta y siete establecimientos públicos de atención de salud que ofrecen servicios de atención de la tuberculosis (TB) y la infección por el virus de la inmunodeficiencia humana (VIH) en Etiopía.
OBJETIVO:
Examinar los obstáculos médicos a la aplicación del tratamiento preventivo con isoniazida (IPT) en las personas infectadas por el VIH.
MÉTODO:
Fue este un estudio transversal que evaluó los factores dependientes del profesional que se asocian con una alta cobertura del IPT, al nivel de los establecimientos.
RESULTADOS:
El análisis bivariante puso en evidencia que la posibilidad de una alta tasa de aplicación del IPT era menor cuando los médicos consideraban que los pacientes padecían efectos negativos de las reacciones adversas al tratamiento (OR 0,18; IC95% 0,04–0,81) y cuando percibían que el IPT aumentaba las tasas de TB multirresistente (OR 0,66; IC95% 0,44–0,98). Se observó que una alta tasa de utilización del IPT exhibía una correlación positiva con la presencia de directrices al respecto en el centro (OR 2,93; IC95% 1,10–7,77) y con la capacitación en materia de atención de la TB y la infección por el VIH (OR 3,08; IC95% 1,11–8,53). En el modelo multivariante, la percepción por parte del médico de una dificultad para descartar la TB activa se correlacionaba de manera negativa con la alta tasa de utilización del IPT (OR 0,93; IC95% 0,90–0,95).
CONCLUSIONES:
El hecho de que los médicos consideren difícil descartar la TB activa en los pacientes infectados por el VIH constituyó) un obstáculo importante a la utilización del IPT según los resultados del presente estudio. El progreso continuo en la aplicación del IPT depende en gran parte de mejorar la capacidad de los profesionales para determinar la aptitud de los pacientes para recibir el tratamiento y de reforzar su confianza en el manejo de los pacientes que reciben el IPT. El hecho de prestar un mayor respaldo e impartir una mejor capacitación a los médicos, además desarrollar nuevas técnicas diagnósticas de la TB, podría fomentar la utilización del IPT por parte de los profesionales.
THERE WERE ~10.4 MILLION new tuberculosis (TB) cases worldwide in 2015, of which people living with the human immunodeficiency virus (PLHIV) accounted for 11%.1 In Ethiopia, HIV/AIDS (acquired immune-deficiency syndrome) and TB collectively resulted in 72.8 (95% uncertainty interval 50.9–101.0) deaths per 100 000 people in 2015, with TB being one the leading causes of mortality and morbidity among PLHIV.2
Studies have demonstrated that isoniazid (INH) preventive treatment (IPT) can effectively prevent TB among PLHIV; the World Health Organization (WHO) recommends ≥6 months of IPT in adolescents and adults living with HIV.3–5 Studies in Brazil and South Africa observed a 76–89% reduction in TB risk among PLHIV receiving antiretroviral therapy (ART) and 6 months of IPT simultaneously.6,7 Another study carried out in Ethiopia reported a reduction in TB incidence by 80% among ART patients on 6 months of IPT, and a 65% reduction in TB incidence among pre-ART patients on 6 months of IPT.8
Several countries, including Ethiopia, have adopted IPT as policy and scaled up IPT implementation, thereby emphasizing routine TB screening of PLHIV at every initial and follow-up visit.9 In efforts to advance intensified case finding and IPT uptake, the WHO recommended a simplified symptom-based TB screening algorithm for PLHIV in resource-limited settings in 2011.10 Despite the push for IPT in policies and guidelines, IPT coverage among newly enrolled HIV patients is estimated to be only 38% globally and 39% in the Africa Region.1 Although Ethiopia began to roll out TB-HIV services in 2006, IPT utilization rates remain low even today. Federal Ministry of Health Ethiopia (FMOH) reports show that during the pilot phase of IPT from 2006 to 2007, an IPT utilization rate of 80% was reached.11 When IPT was more widely implemented, this rate dramatically decreased to 24% by 2008 and further declined to 18.2% by 2012.11
Despite moderate success in IPT implementation in high TB-HIV burden countries, empirical research examining why this policy has failed to become practice is limited. The objective of the present study was to examine the barriers, enablers, and incentives that Ethiopian clinicians face in using IPT among their HIV patients, and evaluate their relationship with facility-level IPT coverage.
METHODS
Study setting and participants
The study was carried out among health facilities providing HIV and TB services supported by the Johns Hopkins Technical Support for Ethiopian HIV/AIDS Initiative (JHU TSEHAI). Regions of JHU TSEHAI operation included Addis Ababa, Benishan-gul Gumuz, Gambella, and the Southern Nations, Nationalities, and Peoples’ Region. All 67 sites supported by JHU TSEHAI at the time were included in the study. The eligibility criteria for clinician participation in the study was at least 2 months of working in TB-HIV care and treatment service provision at a JHU TSEHAI-supported site.
Data collection
Data were collected through interviewer-administered questionnaires among TB-HIV service providers between October 2012 and May 2013. Data on ART and pre-ART patient enrollment, as well as IPT initiation and completion from September 2011 to August 2012, were abstracted from the JHU-TSEHAI facility database for secondary analyses.
Study objectives were communicated to participating sites and informed consent was obtained from all study participants. Approval of the study protocol was granted by the Institutional Review Boards of The Johns Hopkins School of Public Health, Baltimore, MD; Center for Disease Control and Prevention, Atlanta, GA, USA; and the Ethiopian Ministry of Science and Technology, Addis Ababa, Ethiopia. The study protocol was approved by the National Research Ethics Review Committee, Addis Ababa.
Questionnaire
The questionnaire was conceptualized based on predisposing, enabling, and reinforcing factors affecting behavior.12 Questions developed on predisposing factors examined the knowledge, attitudes, and beliefs of clinicians regarding IPT. Questions constructed based on enabling factors explored the clinician perceptions of the availability and accessibility of resources and skills enhancing or inhibiting their use of IPT. Reinforcing factors guided the development of questions examining the influence of peers, patients, and supervisors in encouraging or discouraging IPT use. The questionnaire underwent expert review, translation and back translation, and was then pilot-tested at five facilities in Addis Ababa. The questionnaire was administered by trained JHU TSEHAI staff, who served as clinical mentors to facility staff through routine site visits.
Definitions
The outcome variable of interest was site-level IPT coverage of eligible patients. IPT coverage was calculated based on the proportion of patients having received IPT divided by the total number of eligible IPT patients. A patient who was currently on IPT or had ever been put on IPT based on the patient records database was considered as having received IPT. The number of eligible IPT patients was calculated as 60% of the total number of patients enrolled into pre-ART in the patient records database. The assumption behind this IPT eligibility estimation was that 40% would be ineligible for IPT, with 10–15% of patients having active TB, and approximately 25–30% having some form of contraindications and/or that active TB could not be safely ruled out. Independent factors to explain variation in outcomes included those related to clinician beliefs, attitudes, knowledge, practices, perception of resources available to support IPT implementation, and motivations to use IPT.
Data analysis
To assess if factors and perceived barriers were individually associated with high IPT rates, odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using logistic regression accounting for within-site correlation of responses. All independent variables were evaluated with respect to the IPT rate in unadjusted regression analyses; those significant at the P < 0.2 level were retained for further analysis. Facility type and the total number of health care workers were then examined individually and in conjunction with each predictor in a multivariable logistic regression model. P < 0.05 was considered statistically significant. As the relationship between clinician responses and facility-level IPT uptake rate was analyzed, clustering of clinician responses within each site was controlled for by using STATA commands specifying estimates of the variance–covariance matrix (VCE). The VCE command option in STATA obtained cluster-robust standard errors in all models. All statistical analysis was carried out using STATA 14.0 (StataCorp, College Station, TX, USA).
RESULTS
A total of 133 interviews were conducted at 67 health facilities for the administration of questionnaires; no providers refused participation. However, due to differences in reporting systems and non-availability of data among private facilities, IPT uptake rates could not be calculated for all privately owned sites. Questionnaire data for private facilitates (n = 27) were therefore not included. A total of 106 questionnaire responses from public facilitates were included in the analysis. Characteristics of the participants are shown in Table 1.
Table 1.
Characteristics of clinician respondents at JHU TSEHAI sites (n = 106)
| Characteristic | n (%) |
|---|---|
| Female sex | 45 (42) |
| Qualification | |
| Physician/GP | 23 (22) |
| Health Officer | 19 (18) |
| Nurse (degree) | 12 (11) |
| Nurse (diploma) | 52 (49) |
| Type of facility | |
| Health center | 23 (22) |
| Hospital | 83 (78) |
| Region | |
| Addis Ababa | 25 (24) |
| Benishangul Gumuz | 18 (17) |
| Gambella | 15 (14) |
| SNNPR | 48 (45) |
| Age, years, mean ± SD (range) | 32.8 ± 8.3 (23–57) |
JHU TSEHAI = Johns Hopkins Technical Support for Ethiopian HIV/AIDS Initiative; GP = general physician; SD = standard deviation; SNNPR = Southern Nations, Nationalities, and Peoples’ Region
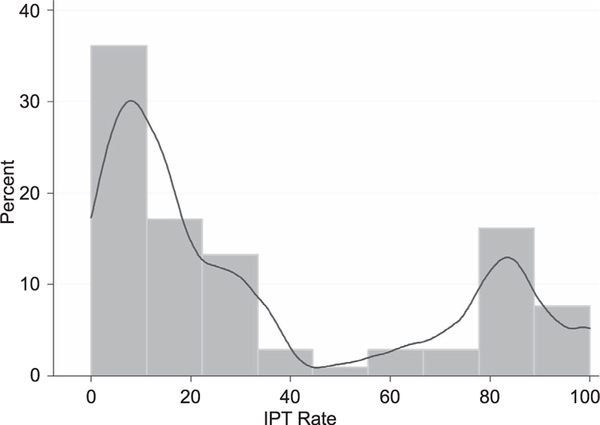
IPT coverage rate across TSHEAI-supported government sites were bimodally distributed with inflection points falling between IPT rates of 0–12.5% and 75–87.5% (Figure). Given the bimodality of the IPT rate distribution, a cut-off point of 40% was used to determine sites with high and low IPT rates. Sites with IPT coverage rates of <40% were considered to be ‘low IPT coverage sites’, whereas those with >40% were considered to be ‘high IPT coverage sites’.
Figure.
IPT uptake rates among TSEHAI sites in 2013 (histogram with Kernel density plot). IPT = isoniazid preventive therapy; JHU TSEHAI = Johns Hopkins Technical Support for Ethiopian HIV/AIDS Initiative.
Factors related to high rates of isoniazid preventive therapy
Bivariate logistic regression analysis showed several factors to be associated with high IPT rates (Table 2). The odds of a high IPT rate were lower among hospitals than health centers (OR 0.12, 95%CI 0.28–0.53). Among predisposing factors, the odds of a high IPT rate were lower if clinicians: perceived that IPT leads to an increase in the rate of multidrug-resistant TB (MDR-TB) (OR 0.66, 95%CI 0.44–0.98); agreed that there were contraindications for IPT (OR 0.41, 95%CI 0.16–0.90); thought that patients were negatively affected by IPT side effects (OR 0.18, 95%CI 0.04–0.81); felt that active TB was difficult to rule out (OR 0.94, 95%CI 0.89–0.97). Enabling factors found to have a statistically significant positive relationship with high IPT uptake were clinicians having received TB-HIV training (OR 3.08, 95%CI 1.11–8.53) and confirmation of IPT guidelines on site (OR 2.93, 95%CI 1.10–7.77). Reinforcing factors were not found to have a significant impact on IPT uptake rates.
Table 2.
Factors associated with high IPT rates on bivariate logistic regression analysis
| Predictor | OR (95%CI) | P value |
|---|---|---|
| Total number of health care workers | 0.13 (0.08–1.16) | 0.71 |
| Predisposing factors (clinician beliefs) | ||
| MoH recommendation for IPT under the HIV program is put into practice at the health facility | 1.02 (0.99–1.08) | 0.20 |
| IPT promotes INH resistance | 0.49 (0.13–1.77) | 0.28 |
| MDR-TB rate increases with IPT | 0.72 (0.47–1.12) | 0.14 |
| Contraindications to IPT exist | 0.13 (0.02–0.55) | 0.05 |
| IPT failure high among patients | 0.99 (0.63–1.57) | 0.99 |
| IPT side effects negatively impact patients | 0.22 (0.05–0.96) | 0.04 |
| Ruling out active TB is difficult | 0.93 (0.90–0.96) | <0.001 |
| Enabling factors | ||
| TB-HIV training ever received | 2.06 (0.65–6.52) | 0.21 |
| Updates on IPT information received from JHU-TSEHAI | 2.42 (0.81–7.24) | 0.11 |
| INH stock out in the last 3 months | 0.56 (0.19–1.65) | 0.29 |
| Guidelines confirmed on site | 2.09 (0.82–5.33) | 0.12 |
| Reinforcing factors | ||
| Supervisors have emphasized improving IPT uptake | 1.42 (0.84–2.41) | 1.91 |
| Agree IPT adherence is poor | 0.71 (0.26–1.89) | 0.50 |
OR = odds ratio; CI = confidence interval; MoH = Ministry of Health; IPT = INH preventive therapy; HIV = human immunodeficiency virus; INH = isoniazid; MDR-TB = multidrug-resistant TB; TB = tuberculosis; JHU TSEHAI = Johns Hopkins Technical Support for Ethiopian HIV/AIDS Initiative.
If the facility type was examined individually and in conjunction with other predictors, it appeared to be a significant differentiator of the IPT rate. Bivariate analyses were therefore adjusted by facility type; three potential barriers to achieving a high IPT emerged (Table 2). These barriers were clinicians: feeling patients were negatively affected by IPT side effects (OR 0.22, 95%CI 0.05–0.96); perceiving that there were contraindications for IPT (0.13, 95%CI 0.02–0.55); having difficulty ruling out active TB in HIV patients (OR 0.93, 95%CI 0.90–0.96).
When further examining all statistically significant factors in a multivariate model, clinician’s perception that active TB was difficult to rule out had a negative association with a high IPT rate (OR 0.93, 95%CI 0.90–0.95). The results of the multivariate logistic regression analysis are given in Table 3.
Table 3.
Factors associated with IPT rates on multivariate logistic regression analysis
| Predictor | OR (95%CI) | P value |
|---|---|---|
| Facility type (hospital) | 0.14 (0.29–0.62) | 0.01 |
| Contraindications to IPT exist | 0.53 (0.20–1.36) | 0.19 |
| Ruling out active TB is difficult | 0.94 (0.89–0.97) | 0.001 |
| IPT side effects negatively impact patients | 0.26 (0.06–1.09) | 0.12 |
OR = odds ratio; CI = confidence interval; IPT = isoniazid preventive therapy; TB = tuberculosis.
DISCUSSION
We discovered that the impression from clinicians that ruling out active TB is difficult was a significant barrier to IPT uptake. This factor has been identified previously as an important barrier to IPT implementation.13,14 The implementation of the WHO four-symptom screening rule has significantly advanced IPT uptake in low-resource settings. In a meta-analysis of 12 observational studies, the sensitivity of the screening rule was found to be 78.9% and specificity 49.6%.5 However, some studies found the algorithm to have lower sensitivity among ART patients (23.8–51.6%) and pregnant HIV-infected women (28.0–42.9%).14–16
In addition to symptom-based screening, it has been reported that clinicians often consider it prudent to conduct sputum culture before IPT initiation where feasible, and that providers do not initiate IPT in cases where they are unsure.17–20 Clinicians in resource-limited settings urgently need additional support and training to determine IPT eligibility, as well as more precise rapid diagnostic tools to increase their confidence in prescribing IPT.
Related to challenges in TB diagnosis is the perception that IPT may fuel MDR-TB. Clinician perception that IPT increased MDR-TB was negatively associated with high IPT rates. Previously reported experiences of TB-HIV program implementers in Ethiopia have indicated that the fear of INH resistance was a challenge to IPT use by health care workers.17 IPT clinical trials among HIV-infected patients in Botswana and South Africa have demonstrated minimal-to-no-increased-risk of INH resistance among IPT patients.21,22 However, further investigation is needed to determine the relationship between IPT and INH risk within operational contexts in which active TB cases can be missed and treatment is often interrupted by drug stock-outs. If clear evidence indicates that the benefits of INH indeed outweigh the risks, and that these risks can be mitigated, it could potentially increase provider certainty about prescribing IPT in Ethiopia.
Clinicians perceived IPT as a necessity for PLHIV but expressed apprehension about the potential contraindications and negative side effects of treatment. Other studies have similarly found that clinicians were hesitant to provide IPT due to toxicity, side effects, and adverse reactions.19,23,24 The existing body of literature reveals that the most serious contraindication to IPT is hepatotoxicity, which occurs in only ≤1% of individuals receiving treatment.25–27 However, given that the prevalence of hepatitis B and C among PLHIV in Ethiopia is relatively high (2.3–14%), concerns about IPT-associated hepatotoxicity are greater among clinicians.19,28,29 According to the Ethiopian Demographic and Health Survey, 53% of men and 48% of women aged 15–49 years consumed alcohol ≥6 days in the past month.30 Both manufactured and homemade alcoholic drinks are consumed in Ethiopia.31 Further studies are required to determine the impact of INH among populations with chronic liver disease. Uncertainty over managing adverse effects remain a challenge due to insufficient training and resources, which limits clinician confidence in providing IPT.14,28
Health centers were more likely to have high IPT uptake than hospitals, a distinction that may reflect the fact that physicians and health officers participating in the study all worked in hospitals, while health centers were primarily run by nurses. Physicians demonstrated greater concern for INH resistance, MDR-TB, as well as potential toxicity among patients. Physicians also reported lower rates of IPT-related training than nurses, which included IPT modules within TB-HIV, basic ART, integrated management of adult illness, or palliative care and nutrition for PLHIV courses. Greater emphasis should be placed on targeting physicians for IPT training and updates by leveraging mentorship, site updates, webinars, and continuing medical education opportunities. Among nurses, further support in monitoring and managing negative IPT side effects, as well as providing greater follow-up and supervision in the area of improving IPT uptake, is also needed. The disparity in on-site confirmation of IPT guidelines between health centers and hospitals points towards the need for increased efforts to ensure that IPT guidelines are readily accessible among hospitals.
The hesitation seen among clinicians regarding MDR-TB, IPT contraindications, and management of side effects highlights an opportunity for improving clinician confidence in IPT provision. Lack of sufficient and timely training as well as limited use of guidelines to support management of patients receiving IPT likely contributed to the clinician concerns found in this study. TB-HIV training and the presence of on-site guidelines were found to positively influence IPT uptake rates. As the body of scientific evidence behind various aspects of IPT is still evolving, frequent updates on scientific findings are especially important to build clinician confidence in IPT provision.
The barriers to provider use of IPT identified in the present study potentially underscore challenges to the uptake of any new and emerging treatment regimens for latent tuberculous infection (LTBI) among PLHIV if key operational challenges in ruling out active TB and provider concerns with MDR-TB persist. Two important areas in advancing provider uptake of LTBI treatment regimens among PLHIV will be consistency of drug supply and availability of accurate point-of-service TB diagnostic tools.
There were several limitations to the findings in this study. Our regression analysis was confined to public/government health facilities due to difficulties in obtaining data to calculate IPT rates from private facilities. Results cannot therefore be extrapolated to all of the TSEHAI facilities providing IPT. However, other than the availability of patient data, there was little difference between public and private facilities in the study sample because identical standard operating procedures were applied and clinicians had received the same type of training. Another limitation was the use of JHU-TSEHAI staff, who also serve as clinical mentors, to administer the questionnaire to clinicians, which may have caused potential bias in responses. However, during the field testing of data collection tools, clinicians provided feedback that they would feel at ease and be able to openly discuss their perceptions of IPT and barriers they faced in using IPT with their clinical mentors. Other limitations included possible inaccuracies and absence of data from patient registries, which were used to calculate IPT rates. Patient adherence and INH stock levels were not directly measured but based instead on clinician reporting.
CONCLUSION
Continued advancement of IPT depends greatly on improving the ability of providers to determine IPT eligibility and care for patients on IPT with greater confidence. Improved clinician support and training as well as development of new TB diagnostic technologies could impact IPT use among providers. Greater emphasis should be placed on provider and patient engagement in ongoing research towards improved IPT strategies to enhance use of new evidence. With the ongoing testing of different IPT implementation models and tools, fostering greater collaboration and information sharing at the local level could result in a broader impact on national development of IPT programs and policies.
Table 4.
Summary of variable data type and question
| Predictor | Data type | Question |
|---|---|---|
| Total number of HCWs in the ART clinic | Continuous variable | Indicate the number of HCWs currently working in the ART clinic |
| Predisposing factors (clinician beliefs) | Based on your experience, do you agree that: | |
| MoH recommendation for IPT under the HIV program is put into practice at the health facility | Categorical variable (yes/no) | The MOH recommends integration of IPT into HIV/AIDS care and treatment programs; do you agree that thisrecommendation is put into practice at your health facility? |
| IPT promotes INH resistance | Categorical variable (yes/no) | IPT promotes drug resistance to INH |
| MDR-TB rate increases with IPT | Categorical variable (yes/no) | MDR-TB rate increases with IPT? |
| Contraindications to IPT exist | Categorical variable (yes/no) | There are contraindications for using IPT among HIV patients? |
| IPT failure high among patients | Categorical variable (yes/no) | IPT failure is high among the HIV patients in my care? |
| IPT side effects negatively impact patients | Categorical variable (yes/no) | My patients are negatively affected by the adverse effects of IPT? |
| Ruling out active TB is difficult | Categorical variable (yes/no) | It is difficult to rule out active TB to decide to start IPT? |
| Enabling factors | ||
| TB-HIV training ever received | Categorical variable (yes/no) | Which of the following trainings/workshop/seminar/ |
| Updates on IPT information received from JHU-TSEHAI | Categorical variable (yes/no) | updates containing IPT information have you ever received? |
| ◻ TB-HIV ◻ Basic ART ◻ IMAI (comprehensive ART) ◻ Palliative care and nutrition ◻ Case review meeting ◻ Site-level updates ◻ Other |
||
| INH stock out in the last 3 months | Categorical variable (yes/no) | Has there been any stock out of INH at your health facility in the last 3 months? |
| Guidelines confirmed on site | Categorical variable (yes/no) | Data collector confirms that a physical copy of the guidelines is at the health facility. |
| Reinforcing factors | ||
| Supervisors emphasize improving IPT | Categorical variable (yes/no) | Is improving IPT uptake rates at your facility discussed by your supervisor? |
| Agree that IPT adherence is poor | Categorical variable (yes/no) | Do you feel that adherence to IPT is poor among patients? |
HCW=health care worker; ART=antiretroviral therapy; MoH=Ministry of Health; IPT=INH preventive therapy; HIV =human immunodeficiency virus; AIDS = acquired immune-deficiency syndrome; INH=isoniazid; MDR-TB=multidrug-resistant TB; TB=tuberculosis; JHU TSEHAI=Johns Hopkins Technical Support for Ethiopian HIV/AIDS Initiative; IMAI = integrated management of adult illness.
Acknowledegements
The authors thank A Nega, the Assistant Data Use Manager, Johns Hopkins Technical Support for Ethiopian HIV/AIDS Initiative (Addis Ababa, Ethiopia), for his assistance with compiling the secondary data set and supporting data entry; and C Thompson, Associate Scientist, Department of Biostatistics at Johns Hopkins University Bloomberg School of Public Health, for her technical support in the quantitative analysis of data. This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (Atlanta, GA, USA) under the terms of 3U2GPS000858.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2016 WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2.Misganaw A, Haregu T N, Deribe K, et al. National mortality burden due to communicable, non-communicable, and other diseases in Ethiopia, 1990–2015: findings from the Global Burden of Disease Study2015. Popul HealthMetr2017; 15: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comstock G W, Ferebee S H, Hammes L M. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis 1967; 95: 935–943. [DOI] [PubMed] [Google Scholar]
- 4.Mancino G, Placido R, Bach S, et al. Infection of human monocytes with Mycobacterium tuberculosis enhances human immunodeficiency virus type 1 replication and transmission to T cells. J Infect Dis 1997; 175: 1531–1535. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Interim policy on collaborative TB/HIV activities WH0/HTM/TB/2004.330, WHO/HTM/HIV/2004.1 Geneva, Switzerland: WHO, 2004. [Google Scholar]
- 6.Getahun H, Kittikraisak W, Heilig C M, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource constrained settings: individual participant data meta-analysis of observational studies. PLOS Med 2011; 8: e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golub J E, Saraceni V, Cavalcante S C, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 2007; 21: 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yirdaw K D, Jerene D, Gashu Z, et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLOS ONE 2014; 9: e104557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Federal Ministry of Health of Ethiopia, Manual for National Tuberculosis and Leprosy Control Programme. Addis Ababa, Ethiopia: Ministry of Health, 2008. [Google Scholar]
- 10.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings WHO/HTM/TB/2011.11 Geneva, Switzerland: WHO, 2011. [Google Scholar]
- 11.Federal Ministry of Health of Ethiopia. Country progress report on the HIV Response, 2014. Addis Ababa, Ethiopia: FMOH, 2014. [Google Scholar]
- 12.Green L W, Kreuter M W. Health program planning: an educational and ecological approach. 4th ed New York, NY: McGraw-Hill, 2005. [Google Scholar]
- 13.Durovni B. Implementing isoniazid preventive therapy for people living with HIV: overcoming the barriers—perspectives of the HIV programme. Cape Town, South Africa: Stop TB Symposium, 2007. [Google Scholar]
- 14.Lester R, Hamilton R, Charalambous S, et al. Barriers to implementation of isoniazid preventive therapy in HIV clinics: a qualitative study. AIDS 2010; 24 (Suppl 5): S45–S48. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad Khan F, Verkuijl S, Parrish A, et al. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS 2014; 28: 1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosgei R J, Szkwarko D, Callens S, et al. Screening for tuberculosis in pregnancy: do we need more than a symptom screen? Experience from Western Kenya. Public Health Action 2013; 3: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaCourse S M, Cranmer L M, Matemo D, et al. Tuberculosis case finding in HIV-infected pregnant women in Kenya reveals poor performance of symptom screening and rapid diagnostic tests. J Acquir Immune Defic Syndr 2016; 71: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melaku Z. TB Screening and IPT Experience from Ethiopia. Poster session presented at the 17th Core Group Meeting of the TB/HIV Working Group, 9–10 November 2011, Beijing, People’s Republic of China. [Google Scholar]
- 19.Mindachew M. Perceived barriers to the implementation of isoniazid preventive therapy for people living with HIV in resource-constrained settings: a qualitative study. Pan Afr Med J 2014; 17: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moolphate S. Barriers to and motivations for the implementation of a treatment programme for latent tuberculosis infection using isoniazid for people living with HIV, in Upper Northern Thailand. Glob J Health Sci 2013; 5: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samandari T, Agizew T B, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment of tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet 2011; 377: 1588–1598. [DOI] [PubMed] [Google Scholar]
- 22.Sterling T, Villarino M, Borisov A, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011; 365: 2155–2166. [DOI] [PubMed] [Google Scholar]
- 23.Eldred L J, Churchyard G, Durovni B, et al. Isoniazid preventive therapy for HIV-infected people: evidence to support implementation. AIDS 2010; 24 (Suppl 5): S1–S3. [DOI] [PubMed] [Google Scholar]
- 24.Smieja M J, Marchetti C A, Cook D J, Smaill F M. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev 2000; (2): CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tedla Z, Nyirenda S, Peeler C, et al. Isoniazid-associated hepatitis and antiretroviral drugs during tuberculosis prophylaxis in HIV-infected adults in Botswana. Am J Respir Crit Care Med 2010; 182: 278–285. [DOI] [PubMed] [Google Scholar]
- 26.Woldehanna S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2004; (1): CD000171. [DOI] [PubMed] [Google Scholar]
- 27.Belyhun Y, Maier M, Mulu A, Diro E, Liebert U G. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis 2016; 16: 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Getahun H, Granich R, Sculier D, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS 2010; 24 (Suppl 5): S57–S65. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Global hepatitis report, 2017. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 30.Fekadu A, Alem A, Hanlon C. Alcohol and drug abuse in Ethiopia: past, present and future. Afr J Drug Alcohol Studies 2007; 6: 39–53. [Google Scholar]
- 31.Central Statistical Agency [Ethiopia] and ICF International. Ethiopia Demographic and Health Survey 2011 Addis Ababa, Ethiopia and Calverton, MD, USA: Central Statistical Agency and ICF International, 2012. [Google Scholar]