Abstract

A novel quaternary cationic pillar[5]arene-modified zeolite (WPA5/zeolite) was prepared via charge interaction between the cationic WPA5 and natural zeolite and characterized by scanning electron microscopy (SEM), Fourier transform infrared absorption spectroscopy, X-ray diffraction, solid-state nuclear magnetic resonance, and thermogravimetric (TG) analysis. The effects of zeolite particle size, WPA5 concentration, adsorption time, initial concentration, and pH on the removal of methyl orange (MO) were studied. The SEM and XRD results revealed a strong interaction between WPA5 and natural zeolite, and the modified composites showed novel microscopic morphology and structural properties. TG analysis indicated excellent thermal stability of the composite. MO was removed via electrostatic adsorption, and the removal efficiency was 84% at an initial concentration of 100 mg/L. Increase in the initial dye concentration enhanced the adsorption capacity of WPA5/zeolite and decreased the removal of MO. Based on the adsorption kinetics, the pseudo-second-order model (R2 = 0.998) described the kinetic behavior of MO on WPA5/zeolite. In addition, UV and fluorescence spectra revealed that MO and WPA5 are complexed by a 1:1 complex ratio, and the binding constant between them was 12 595 L·mol–1. NMR and molecular docking also verified their interaction. Therefore, the potential application of the prepared composite includes removal of organic anionic dyes.
1. Introduction
The rapid growth of cosmetic, rubber, plastic, textile, and paper industries has increased the severity of water and environmental pollution.1 Wastewater contaminated with organic dye is recognized as one of the major sources of industrial pollution worldwide.2 Each year, 700 000 tons of dyes are consumed by the textile industry. Therefore, untreated effluents discharged by the printing and dyeing industry are one of the important causes of water pollution.3 The composition of printing and dyeing wastewater is complex and extremely toxic to the ecosystem, and it is highly difficult to remove the organic dye molecules.4,5 Methyl orange (MO) (C14H14N3NaO3S; MO; Figure 1), an anionic azo dye, is carcinogenic and mutagenic, in addition to triggering shock, increasing heart rate, inducing jaundice, vomiting, and tissue necrosis.6−10 Treatment methods for dye removal from water include oxidation, membrane separation, coagulation, adsorption, chemical reduction, and biological treatment.11−13 Adsorption is economical and effective because of its simplicity and high efficiency.14
Figure 1.
Molecular structures of (a) WPA5, (b) MO dye, (c) zeolite, and the adsorption mechanism of MO using modified zeolite.
Natural zeolite has been widely used in the aqueous treatment of environmental contaminants because of its wide variety, abundant reserves, low price, and simplicity of processing technology.15,16 Zeolite has a unique structure comprising an aluminosilicate skeleton and abundant intracrystalline pores, resulting in strong ion exchange and adsorption properties.17,18 However, zeolites exhibit a permanent negative electrical structure due to isomorphous replacement, which results in weak adsorption of organic anions. Therefore, modification of natural zeolites has been attempted to address the issue.19,20
Macrocyclic compounds playing an increasingly important role in the treatment of Pillararene was initially discovered by Ogoshi in 2008.21 It has a tubular morphology compared with other macrocyclic molecules such as crown ether,22 cyclodextrin,23−25calixarene,26,27 and cucurbituril.28 Pillararenes have been widely used in gas adsorption,29,30 wastewater treatment,31−33 drug transport,36−39 and molecular recognition40−43 due to their easy modification, simplicity of preparation, and optimal performance.34,35
In this work, we report the preparation and characterization of water-soluble pillar[5]arene-modified zeolite (WPA5/zeolite). Subsequently, the adsorption of modified zeolite on MO was studied (Figure 1). In addition, the effects of zeolite particle size, initial dye concentration, adsorption time, and pH on the adsorption process were investigated. To the best of our knowledge, studies investigating WPA5/zeolite for MO dye removal have yet to be reported. Therefore, the primary aim of this work is the development of a novel method of zeolite modification, with potential application in the field of water pollution treatment.
2. Results and Discussion
2.1. SEM and Optical Microscopic Analysis
The morphologies of WPA5, zeolite, and their composites were investigated via scanning electron microscopy (SEM) (Figure 2). The microstructure of WPA5 was characterized by a planar structure with irregular lines. The natural zeolite exhibited an uneven sheet-like structure, with clearly visible crevice lines between the sheets. However, the WPA5/zeolite appeared as a large, irregular, and spherical structure, and the original sulcus of natural zeolite was filled. The WPA5/zeolite varied in size and shape with WPA5 and natural zeolite, which confirmed the formation of novel composite materials.
Figure 2.
SEM images of (a) WPA5, (b) WPA5/zeolite, and (c) zeolite.
The morphologies of WPA5/zeolite and natural zeolite after adsorption of MO were determined using optical photomicroscopy (Figure 3). As shown in the figure, the WPA5/zeolite was dyed deep orange after MO adsorption. However, the permanent negative charge of natural zeolite resulted in an electrostatic repulsion between the MO and the zeolite. Therefore, the natural zeolite displayed a light orange color upon MO adsorption.
Figure 3.

Optical microscopic images of natural zeolite and WPA5/zeolite after MO adsorption.
2.2. FTIR Analysis
Figure 4 shows the Fourier transform infrared absorption spectroscopy (FTIR) spectra of WPA5, zeolite, and their composite. The peak at 474.00 cm–1 represents the bending vibration of the Si–O bond, and the peak at 539.42 cm–1 denotes the telescopic vibration of the Si–O–Al. The peak at 794.46 cm–1 indicates the bending vibration of −OH on Si–OH, and the peak at 1098.84 cm–1 represents the stretching vibration of Si–O– in the zeolite structure. The characteristic peak of −OH is observed at 3433.87 cm–1, and the peak at 3619.86 cm–1 indicates stretching vibration generated by −OH combined with Si. In addition, the peak at 1209.84 cm–1 in the spectrum of WPA5 suggests a telescopic vibration of the C–N bond. The peak at 1404 cm–1 corresponds to the bending vibration of CH3–N+, and the peak at 1484.36 cm–1 denotes shear vibration of the −CH2–N+ bond. The vibrations of CH3–N+ and −CH2–N+ in WPA5 are found in the FTIR spectrum of the modified zeolite but not in the natural zeolite. Therefore, this phenomenon suggests successful synthesis of WPA5-modified zeolite.
Figure 4.
FTIR spectra of (a) WPA5, (b) WPA5/zeolite, and (c) zeolite.
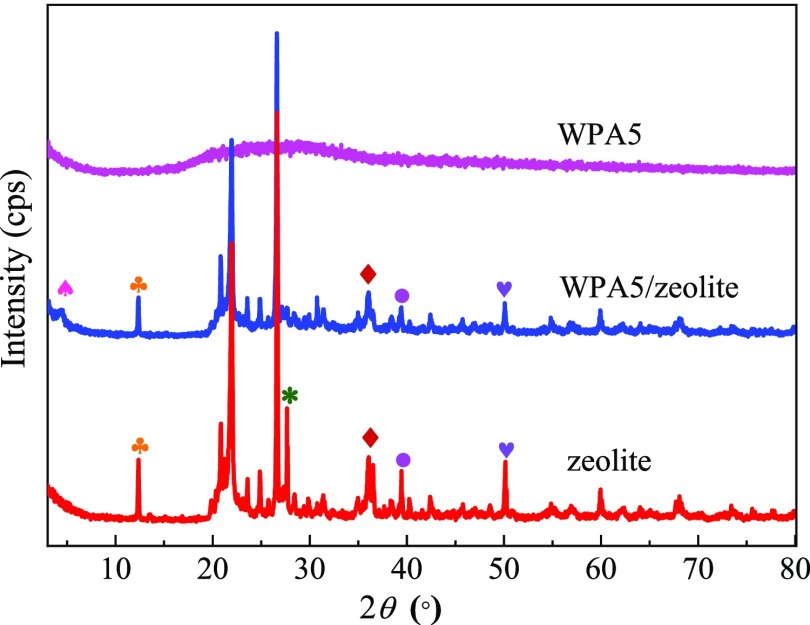
2.3. XRD Analysis
Figure 5 displays the X-ray diffraction (XRD) patterns of WPA5, zeolite, and the WPA5/zeolite composite. The XRD pattern of WPA5 reveals its amorphous structure. The natural zeolite yields major crystal diffraction peaks at 12.36°, 20.85°, 21.97°, 23.59°, 24.86°, 26.62°, 27.65°, 36.07°, 36.52°, 39.45°, 50.15°, and 59.93°. However, the significant characteristic peaks of natural zeolite at 27.65° and 36.52° were not observed in the WPA5/zeolite composite, and the major peaks of zeolite were weakened in the composites, which indicated the strong interaction between WPA5 and zeolite.
Figure 5.
X-ray diffraction patterns of WPA5, WPA5/zeolite, and zeolite.
2.4. SSNMR Analysis
The formation of WPA5/zeolite was further confirmed using 13C SSNMR analysis (Figure 6). The peaks located between 10 and 70 ppm (α) were assigned as methyl (−CH3) and methylene (−CH2−) in WPA5 (Figure 6a), and the peaks located between 110 and 210 ppm (β, γ) can be resolved into the sp2 carbons of the phenyl rings in the WPA5. The carbon peaks in WPA5 can be clearly found in the 13C SSNMR spectrum of WPA5/zeolite (Figure 6b), and no carbon signal is detected in natural zeolite (Figure 6c). These phenomena strongly illustrate the successful preparation of WPA5-modified zeolite.
Figure 6.

13C SSNMR characterization of (a) WPA5, (b) WPA5/zeolite, and (c) zeolite. (d) 13C NMR characterization of WPA5.
2.5. TG Analysis
The thermogravimetric (TG) curves of WPA5, zeolite, and WPA5/zeolite are shown in Figure 7. The melting point of WPA5 ranges from 271.2 to 271.8 °C, suggesting small amounts of bound and free water in WPA5. A weight loss of 11.84 wt % occurred initially when the temperature increased to 290 °C. A major weight loss of WPA5 (64.88 wt %) in the region of 300–390 °C suggested degradation. The final weight loss at 390–780 °C (28.49 wt %) revealed the decomposition of WPA5. However, the entire warming process had little effect on the weight loss (4.05%) of natural zeolite. Notably, the total weight loss of the WPA5/zeolite was 8.89 wt %, indicating the loading of a specific WPA5 mass onto the zeolite. In addition, TG analysis demonstrated that zeolite increased the thermal stability of WPA5.
Figure 7.
TG curves of WPA5, WPA5/zeolite, and zeolite.
2.6. Adsorption Study
2.6.1. Standard Curve of MO
The standard curve of MO is shown in Figure 8. It was calculated using the equation C = (A + 0.02331)/0.07891, and it has a correlation coefficient of R = 0.9999.
Figure 8.
Standard curve of MO.
2.6.2. Effect of Zeolite Particle Size
The percent removal of MO by zeolites and WPA5/zeolite of different particle sizes is shown in Figure 9. The removal of MO by natural zeolite was very limited, and the average removal efficiency was about 4%. The adsorption of MO by natural zeolites was greatly improved after modification with WPA5. Doping of WPA5 onto the surface and pores of the zeolite via charge interaction resulted in a positive charge and facilitated the removal of the anionic pollutant MO. When the particle size was reduced from 10 to 30 mesh, the adsorption of MO by the WPA5/zeolite was continuously increased to a maximum of 85% at 30 mesh. When the particle size was decreased, the removal of MO by the WPA5/zeolite also declined. The reduced particle size increased the specific surface area of the zeolite, which provided additional ion exchange sites for the binding of WPA5 molecules to the zeolite. When the particle size was reduced, the increased specific surface area of the zeolite provided additional ion exchange sites, facilitating the binding of WPA5 molecules to the zeolite. However, the decreased space was not conducive to WPA5 binding to the surface of the zeolite and thus the percent removal of MO was reduced.
Figure 9.

Effect of zeolite particle size on MO adsorption.
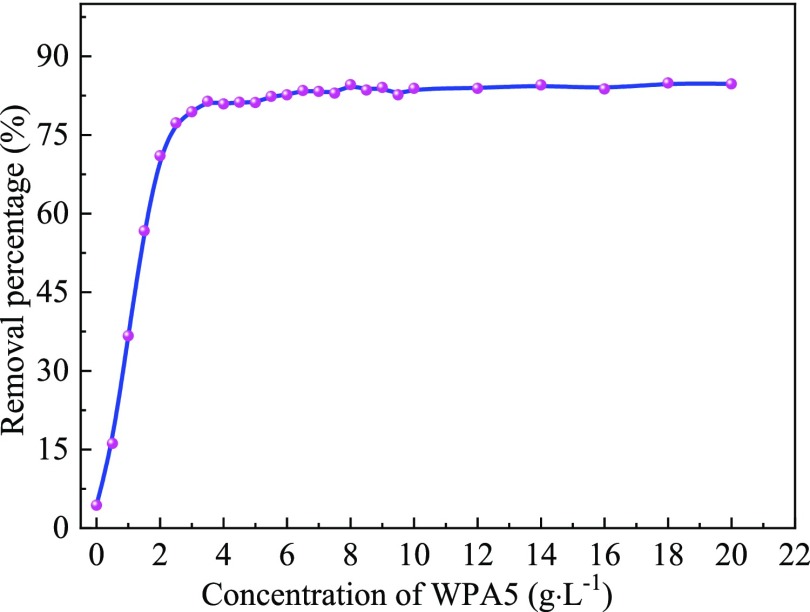
2.6.3. Effect of WPA5 Concentration
The effect of different concentrations of WPA5-modified natural zeolite (30 mesh) on the removal of MO is shown in Figure 10. The removal efficiency of MO by natural zeolite was 4.4% and that of MO by WPA5/zeolite dissolved in 0.5 g/L WPA5 solution was 16.2%. With the increased WPA5 concentration, the percent removal of MO by WPA5/zeolite was also increased. When the WPA5 concentration was 3.5 g/L, the removal of MO by the WPA5/zeolite reached 81.4%. As the WPA5 concentration continued to increase, the removal of MO by the modified zeolite tended to increase slowly, but this increase was not significant. When the WPA5 concentration was 8.0 g/L, the removal of MO by the WPA5/zeolite reached 84.6%. Based on the dosage of WPA5 and the percent removal of MO, the WPA5 solution at a concentration of 8.0 g/L was selected to modify the zeolite in a subsequent study.
Figure 10.
Effect of different concentrations of WPA5 on the adsorption of the MO dye.
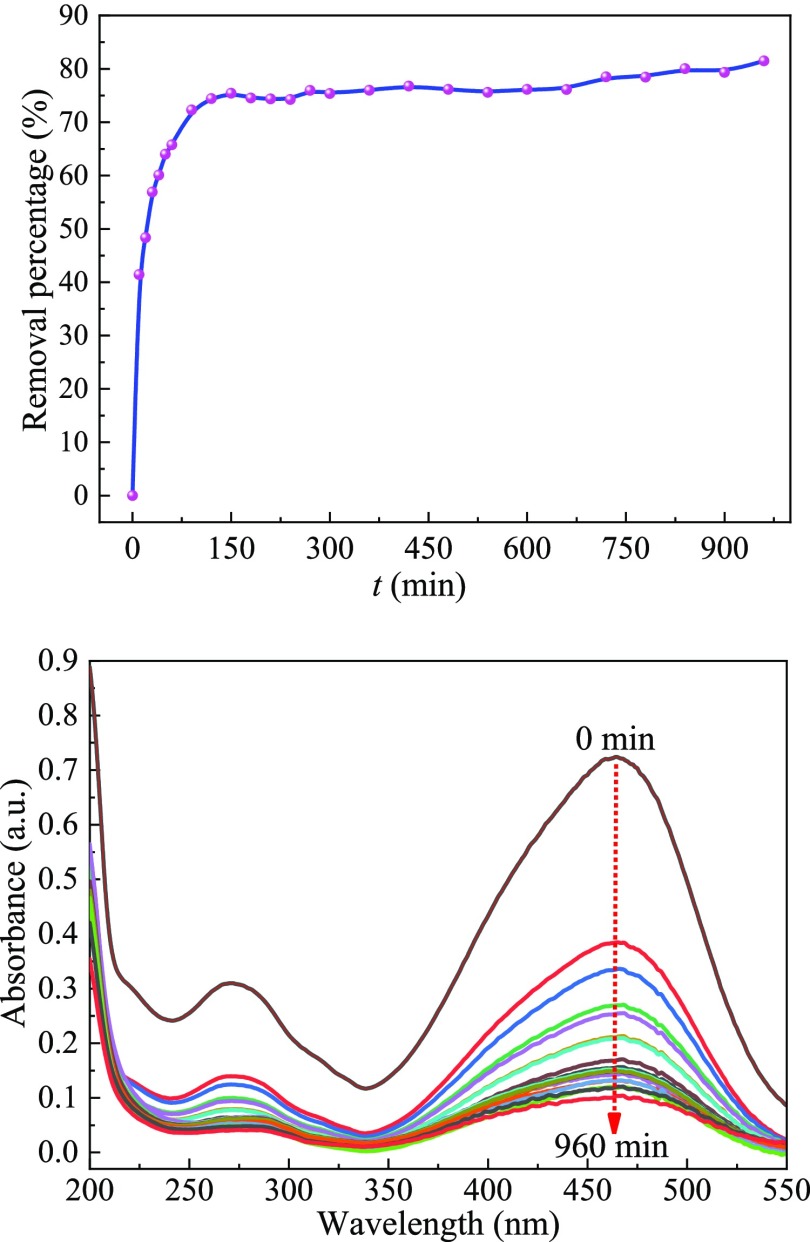
2.6.4. Effect of Contact Time
The effect of adsorption time on MO removal is shown in Figure 11. In general, as the adsorption time was prolonged, the percent removal increased. The rapid adsorption from 0 to 150 min was partly attributed to insufficient internal diffusion resistance and a substantial number of adsorption sites. When the adsorption time increased to 150 min, the removal of MO by the modified zeolite was 75.45%. After 150 min, the concentration of MO gradually decreased, which led to a decrease in the concentration gradient between the solution and the zeolite surface. Hence, the diffusion resistance was increased, and the adsorption rate declined. The percent removal of MO by the modified zeolite increased very slowly and reached 81.51% in 960 min.
Figure 11.
Effect of contact time on the adsorption of the MO dye.
2.6.5. Effect of Initial Concentration
The initial concentration of MO plays an important role in the removal of the contaminant by allowing the dye molecule to overcome the mass transfer resistance generated from the aqueous to the solid phase. As shown in Figure 12, different initial concentrations of MO solution affect the dye removal and adsorption capacity. It is apparent from the figure that the percent removal of MO by the modified zeolite decreases as the initial concentration increases due to the decreased number of active sites in the modified zeolite.
Figure 12.
Effect of the initial dye concentration on MO adsorption.
2.6.6. Effect of pH
Solution pH is a meaningful parameter in the adsorption of pollutants. It changes the efficiency of MO adsorption by regulating the surface charge and dissociation of the zeolite. The effect of pH on the removal of MO is shown in Figure 13. It is apparent that the WPA5/zeolite showed an “M” shape for the percent removal of MO in the pH range of 2–12 and reached a maximum at pH 5. Subsequently, the percent removal declined to a minimum at pH 7. However, as the pH increased from 7 to 9, the removal was enhanced and reached a maximum at pH 9. However, as the pH continued to increase, the surface of the WPA5/zeolite attracted OH– ions more efficiently than MO with a large molecular structure. This factor mainly contributed to the reduction of MO clearance by WPA5/zeolite at a high pH.
Figure 13.
Effect of pH on MO adsorption.
2.7. Adsorption Kinetics
Two kinetic models (pseudo-first-order and pseudo-second-order models) were used to study the adsorption mechanism of modified zeolites. Pseudo-first-order models have been widely used to study the adsorption behavior of solid/liquid systems. The linear form of the model is represented by the following equation
| 1 |
In the above equation, the term qt (mg·g–1) denotes adsorption capacity at time t, qe (mg·g–1) represents adsorption capacity at equilibrium, and k1 (min–1) is the pseudo-first-order-rate constant.
The linear form of the pseudo-second-order model is represented by the following formula
| 2 |
The second-order-rate constant k2 and the equilibrium adsorption capacity qe can be determined from the t/qt versus t curve.
The linear plots of pseudo-first-order and pseudo-second-order models for MO adsorption by WPA5/zeolite are shown in Figure 14. The correlation coefficients are listed in Table 1. The goodness of fit can be determined by fitting the linear regression coefficient R of the straight line. In addition, the degree of closeness between the adsorption capacity obtained via comparison of qe,exp and qe,cal obtained experimentally can also be used as a basis for the determination of the goodness of fit of the kinetic model. The correlation coefficient R (0.966) of the pseudo-first-order model was lower than that of the pseudo-second-order model, and there was a large deviation between its theoretical adsorption capacity qe,cal (2.08) and the experimental adsorption capacity qe,exp (4.04). The R2 value of the pseudo-second-order model was 0.996, and the pseudo-second-order theoretical adsorption capacity qe,cal (3.88) was close to the experimental value. Therefore, the pseudo-secondary-model is more efficient in describing the adsorption behavior of MO on WPA5/zeolite.
Figure 14.
Adsorption kinetics of MO adsorbed by WPA5/zeolite: (a) pseudo-first-order model and (b) pseudo-second-order model.
Table 1. Adsorption Kinetic Parameters of MO on WPA5/Zeolite.
| pseudo-first-order |
pseudo-second-order |
|||||
|---|---|---|---|---|---|---|
| exp qe,exp (mg·g–1) | K1 (min–1) | qe (mg·g–1) | R2 | K2 (g·mg–1·min–1) | qe (mg·g–1) | R2 |
| 4.04 | 0.0143 | 2.08 | 0.966 | 2.96 | 3.88 | 0.999 |
2.8. Adsorption Isotherm
Adsorption thermodynamics studies are important in delineating the adsorbent–adsorbate interactions and the design of adsorption systems. Langmuir and Freundlich isotherms have been widely used because of fewer parameters and simplicity, with a high degree of fitness to the adsorption behavior of solid–liquid systems.
The Langmuir equation is expressed as follows
| 3 |
The equation for the linear change of eq 3 is as follows
| 4 |
where qe is the equilibrium adsorption capacity of the adsorbent (mg·g–1), Ce denotes the equilibrium concentration of the adsorbate (mg·L–1), KL represents the Langmuir constant (L/mg), and qm refers to the maximum adsorption capacity (mg·g–1).
The separation factor or equilibrium parameter is expressed by the following formula
| 5 |
In the above equation, if RL > 1, the isotherm nature is unfavorable; linear if RL = 1; favorable if 0 < RL < 1; and irreversible if RL = 0.
The Freundlich isotherm model is an empirical formula used for the determination of multimolecular layer adsorption, which has a certain degree of generality. The most common type of adsorption isotherm equation is as follows
| 6 |
The linearized form of the Freundlich isotherm model is as follows
| 7 |
Figure 15 illustrates the Langmuir and Freundlich isotherms for the adsorption of MO on modified zeolites. The correlation coefficients are listed in Table 2. The correlation coefficient of the Langmuir model (0.98) was greater than that of the Freundlich model (0.968). The results show that the Langmuir model can be used to describe the adsorption of MO on WPA5/zeolite more effectively than the other model.
Figure 15.
(a) Langmuir and (b) Freundlich isotherm models for MO adsorption.
Table 2. Langmuir and Freundlich Isotherm Parameters.
| Langmuir isotherm |
Freundlich isotherm |
|||||
|---|---|---|---|---|---|---|
| exp qe,exp (mg·g–1) | KL | qmax (mg·g–1) | R2 | n | KF (mg·g–1) | R2 |
| 4.04 | 0.0482 | 8.33 | 0.98 | 2.50 | 1.07 | 0.968 |
2.9. Analysis of Interaction between WPA5 and MO
The UV spectrum titration diagram of WPA5 and MO is shown in Figure 16a. Assuming a 1:1 stoichiometry for the MO/WPA5 inclusion complex, the inclusion complexation of MO with WPA5 could be expressed by eq 8. Then, the stability constant (Ks) can be calculated from eq 9. We can obtain eq 10 from eq 9. Finally, Ks can be obtained from the investigation of the sequential vary of absorption (ΔA) at WPA5 concentrations, by a nonlinear least squares method according to the curve-fitting eq 10.
| 8 |
| 9 |
| 10 |
where [MO/WPA5], [MO], [WPA5], [MO]0, and [WPA5]0 are the equilibrium concentrations of the MO/WPA5, MO, WPA5, and the original concentrations of MO and WAP5, respectively (mM); ΔA indicates the change in absorbance of WPA5 in the presence or absence of MO; and Δε is the differential molar extinction coefficient of WPA5 in the absence and presence of MO.
Figure 16.
(a) Ultraviolet titration of the WPA5 and MO in water: 4 × 10–5 mol·L–1 WPA5, 0.05–1 equiv of MO. (b) Fluorescence spectroscopy titration of the WPA5 and MO in water: 2 × 10–5 mol·L–1 WPA5, 0.1–1.5 equiv of MO, 285 nm excitation wavelength, slit = 5/5 nm. (c) Job’s plot for determining the stoichiometry in the complex of the WPA5 and MO in water. (d) Variation of the absorption of WPA5 upon addition of MO in water: 3 × 10–5 mol·L–1 MO, 0.2–3 equiv of WPA5.
We obtained the complex stability constant for the MO/WPA5 via a nonlinear least squares curve-fitting method. The stability constant Ks and Gibbs free energy ΔG of the inclusion complex of WPA5 and MO in the aqueous phase are 12 595 L·mol–1 (log Ks = 4.0995) and −23.403 kJ·mol–1, respectively. It can be seen from the inset in Figure 16a that there is a good linear match between the experimental value and the theoretical value, which shows the 1:1 stoichiometry of the MO/WAP5 inclusion complex.
As shown in Figure 16b, WPA5 has a fluorescence peak at 235 nm. With the addition of different concentrations of MO, the fluorescence of WPA5 gradually decreases. This indicates that the fluorescence properties of WPA5 are significantly changed after complexing with MO. Furthermore, Job’s method was employed to obtain the stoichiometry of MO and WPA5 via the fluorescence spectroscopy. In Figure 16c, the value of the max mole fraction of MO in this work is 0.5, which strongly proves the formation of the MO/WPA5 inclusion complex with a stoichiometry of 1:1. The molecular docking diagram of WPA5 and MO is shown in Figure 17. MO ran through the cavity of the WP5A and was at the geometric center of WPA5. Then, the entire MO molecule was encapsulated by WP5A, at which time the MO molecule was docked on the inner wall of WP5A, eventually forming an MO/WP5A coordination complex.
Figure 17.

Optimal conformation formed by docking MO to the cavity of WPA5.
1H NMR data confirmed the MO and WPA5 complexation in water. As shown in Figure 18, after adding the equivalent amount of MO to WPA5, the signal of the proton signal of the host and the guest exhibits a significant shift or signal broadening effect. Thus, MO should be more deeply incorporated into the macrocycle cavity of WAP5. Furthermore, the 2D NMR 1H–1H NOESY of the complexes confirms host–guest complexation (Figure 19). The aromatic protons of MO (D, C, E) have cross-peaks with H1, H3, and H4 of WPA5. The aromatic protons of MO (B) have cross-peaks with H1 and H3 of WPA5. Thus, we can conclude that the MO/WPA5 inclusion complex is formed.
Figure 18.
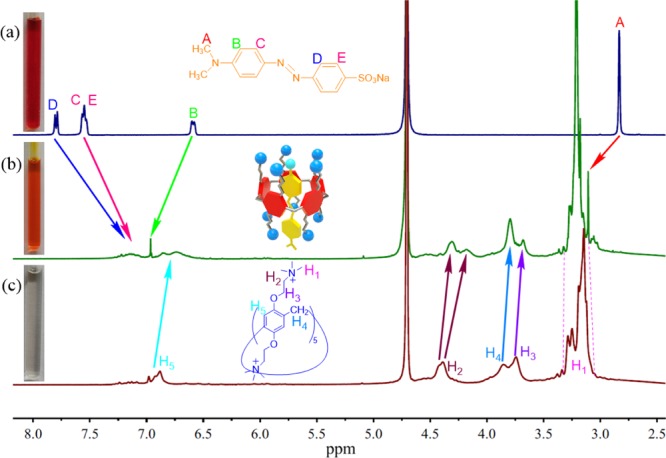
1H NMR spectra (D2O): (a) MO (0.015 mol·L–1); (b) MO (0.015 mol·L–1) + WPA5 (0.015 mol·L–1); (c) WPA5 (0.015 mol·L–1).
Figure 19.
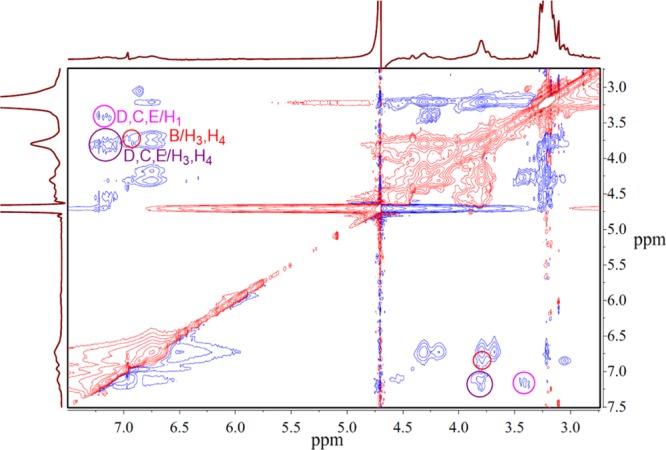
2D NMR 1H–1H NOESY analysis of MO with WPA5 in D2O. The concentrations of the host and the guest are 0.015 mol·L–1.
The removal ability of pure WPA5 to MO is shown in Figure 16d. The UV absorption of MO exhibits a blue shift and small decreases with the addition of WPA5. However, the modified zeolite does not undergo a blue shift when adsorbing MO, and the absorbance is remarkably lowered (Figure 11). In addition, WPA5 is water-soluble; it does not remove the MO from the aqueous phase and does not achieve the purpose of treating sewage. This experiment reveals the correctness of the experimental scheme for the adsorption of MO by WPA5-modified zeolite.
3. Conclusions
This study focused on the development of a novel, environmentally friendly WPA5/zeolite for the adsorption of MO from aqueous solutions. The SEM, XRD, FTIR, and SSNMR characterizations indicated successful adsorption of WPA5 on the surface of the zeolite. TG examinations confirmed the thermal stability of the WPA5/zeolite. The effects of zeolite particle size, WPA5 concentration, adsorption time, initial concentration, and pH on the percent removal efficiency of MO were also investigated. The kinetic and adsorption data indicated that the adsorption of MO on the WPA5/zeolite could be best described using linear forms of pseudo-second-order models. In addition, UV and fluorescence spectroscopy, NMR, and molecular docking provided an in-depth analysis of the mechanism by which WPA5 interacts with MO. The WPA5/zeolite is a promising candidate for the removal of MO.
4. Experimental Section
4.1. Materials
Natural zeolite was purchased from Hengnuo Filter material Co. Ltd. (Henan Province, P.R. China). MO, hydroquinone bis(2-hydroxyethyl)ether, paraformaldehyde, tetrabromomethane, triphenylphosphine, boron trifluoride etherate (98%), and trimethylamine (30%) were purchased from Shanghai Titan Technology Co. Ltd. All of the reagents used were of analytical grade (A. R.). All of the aqueous solutions were prepared using ultrapure water.
4.2. Synthesis of WPA5
WPA5 was synthesized as described in the Supporting Information (Figure 1).44 A solution of CBr4 (33.46 g, 100.90 mmol) in acetonitrile (250 mL) was added dropwise to the acetonitrile (50 mL) of hydroquinone bis(2-hydroxyethyl)ether (10.0 g, 50.45 mmol) and PPh3 (36.46 g, 100.90 mmol) under an atmosphere of nitrogen and reacted at room temperature for 4 h to obtain compound 2 (82%). To a solution of compound 2 (3.24 g, 10.00 mmol) in 1,2-dichloroethane (100 mL), paraformaldehyde (0.90 g, 30 mmol) was added under nitrogen atmosphere; this was followed by the addition of BF3·OEt2 (1.39 mL, 10 mmol) to the solution. The mixture was stirred at room temperature for 6 h, resulting in a green solution. After the solvent was concentrated in vacuo, the residue was purified by column chromatography on silica gel with petroleum ether/dichloromethane (1:1 v/v) as the eluant to yield compound 3 (40%) as white powder. Compound 3 (0.4813 g, 0.2865 mmol) and excessive trimethylamine (3.2 mL, 11.46 mmol) were added to ethanol (30 mL). The solution was refluxed for 36 h. The solvent was concentrated in vacuo, and 10 mL of deionized water was added. After filtration, a clear solution was obtained. Finally, the water was concentrated in vacuo to yield compound 4 as a colorless solid (91%), with the following spectroscopic features: 1H NMR (400 MHz, D2O) δ (ppm): 6.89 (s, 10H), 4.49 (s, 10H), 3.84 (s, 20H), 3.24 (s, 90H); 13C NMR (100 MHz, D2O) δ (ppm): 149.30, 129.89, 116.55, 64.91, 63.49, 54.13, 29.57.
4.3. Preparation of the WPA5/Zeolite Composite
The zeolite was added to a WPA5 solution (10 mL of WPA5 aqueous solution per 1 g of zeolite). The mixture was shaken at room temperature for 24 h; this was followed by filtration of the solution. The WPA5/zeolite composite was repeatedly washed with ultrapure water (40 mL × 3) and finally dried at 50 °C to constant weight.
4.4. Characterization
1H and 13C NMR spectra were recorded on a Bruker AVANCE 400 nuclear magnetic resonance spectrometer using tetramethylsilane as the internal standard. SSNMR data were recorded on JNM-ECZ600R. The absorbance was measured using an Agilent 8453 UV–vis spectrometer. The fluorescence data were recorded on a Cary Eclipse fluorescence spectrophotometer. The FTIR spectra were measured with a Thermo Nicolet Avatar 360 spectrometer using the KBr pellet. XRD patterns of powder samples were recorded using a D/Max-3B diffractometer with Cu Kα radiation (λ = 0.15406 nm, 100 mA, 40 kV). The scan rate was 5°/min, and the range of 2θ = 0.02° varied between 3° and 80°. The sample microstructure was analyzed using a FEL Quanta 200 scanning electron microscope. Optical microscopy was performed using SOPTOP CX40. TG measurements were conducted with NETZSCH STA 449F3 by increasing the temperature to 900 °C at a heating rate of 10 °C/min in a dynamic nitrogen atmosphere.
4.5. Adsorption Experiments
4.5.1. Standard Curve of MO
Aliquots of 100 mL containing 150 mg/L MO solution were configured accurately, and the following volumes of MO solution were added to a 10 mL volumetric flask: 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.8 mL. After ensuring a constant volume, the flasks were shaken. The absorbance was measured at the maximum absorption wavelength (467 nm).
4.5.2. Effect of Zeolite Particle Size
The zeolite was divided into different sizes, 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 mesh, using a standard sampling sieve. A 10 g/L WPA5 solution was added to the zeolite and shaken for 24 h; this was followed by removal of the supernatant and washing with ultrapure water. Finally, the zeolite was dried at 50 °C to obtain a constant weight. Zeolite particles of different sizes and weighing 0.5 g each were transferred to a 50 mL stoppered conical flask containing the WPA5/zeolite mixture; this was followed by the addition of 25 mL of the 100 mg/L MO solution and shaking at room temperature for 12 h. After standing for 1 h, the UV absorption spectrum was measured, and the concentrations of MO before and after adsorption were determined from the standard curve of MO.
The adsorption amount (qe) and removal percentage (R) of the modified zeolite for MO were calculated using the following equations
| 11 |
| 12 |
4.5.3. Effect of WPA5 Concentration
A series of WPA5 solutions with concentrations ranging from 0.5 to 20 g/L (0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5, 8, 8.5, 9, 9.5, 10, 12, 14, 16, 18, and 20 g/L) were configured. The zeolite was modified with different concentrations of WPA5. It was washed repeatedly with ultrapure water and dried at 50 °C to constant weight. Zeolite modified with different concentrations of aromatic hydrocarbons was accurately weighed to 0.5 g in a 50 mL conical flask. This was followed by the addition of 25 mL of the 100 mg/L MO solution and shaking at room temperature for 12 h. The absorbance was measured after centrifugation at 3000 rpm for 10 min.
4.5.4. Effect of Contact Time
A batch of modified zeolite was prepared using 8 g/L of WPA5 solution and 30 mesh zeolite. About 0.5 g of zeolite was added into a 50 mL stoppered conical flask and mixed with 25 mL of a 100 mg/L MO solution. The mixture was oscillated at room temperature for different times (10, 20, 30, 40, 50, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330, 360, 420, 480, 540, 600, 660, 720, 780, 840, 900, and 960 min), and the removal percentage of MO by the modified zeolite was calculated according to the method described in Section 4.5.2.
4.5.5. Effect of Initial Concentration
After configuring a series of concentrations of MO solution (20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 250, and 300 mg/L), the adsorption experiments were conducted by the addition of 0.5 g of modified zeolite.
4.5.6. Effect of pH
A specific concentration of MO solution was obtained by adjusting the pH (2–12) of the aqueous solutions using HCl and NaOH.
4.6. Interaction between WPA5 and MO
4.6.1. Determination by UV and Fluorescence Spectroscopy
An in-depth study of the host–guest interaction between WPA5 and MO helps us to understand the adsorption mechanism. To quantify the complexation of the MO by WPA5, the stability constants of the MO/WPA5 complexes formed in water were estimated by ultraviolet titration. The concentration of WPA5 was held constant at 0.04 mM. Then, an appropriate amount of MO was added, and the final concentrations varied from 0 to 0.04 mM (MO: 0, 0.002, 0.004, 0.006, 0.008, 0.01, 0.012, 0.014, 0.016, 0.018, 0.02, 0.022, 0.024, 0.026, 0.028, 0.03, 0.032, 0.034, 0.036, 0.038, and 0.04 mM in water). In addition, the fluorescence properties of the supramolecular system of MO with WPA5 were investigated by fluorescence spectroscopy titration. The concentration of WPA5 was held constant at 0.02 mM. Then, an appropriate amount of MO was added, and the final concentrations varied from 0 to 0.03 mM (MO: 0, 0.002, 0.004, 0.006, 0.008, 0.01, 0.012, 0.014, 0.016, 0.018, 0.02, 0.022, 0.024, 0.026, 0.028, and 0.03 mM in water). The spectral data were recorded after waiting for 30 min. The UV spectroscopy measurements were done in the 280–500 nm spectral range. For fluorescence tests, the excitation wavelength was 285 nm. The excitation and emission slits were set at 5/5 nm, respectively. All of the experiments were carried out in triplicate.
4.6.2. Job’s Plot Measurements
A solution of WP5A and MO at a concentration of 0.02 mM was prepared using water. First, 5.0, 4.5, 4.0, 3.5, 3.0, 2.5, 2.0, 1.5, 1.0 0.5, and 0 mL of the WP5A solution were taken and transferred to 5 mL volumetric flasks. Then, 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5 mL of the MO solution were added to each vial of WP5A solution, making the total volume of each vial 5 mL. After shaking, fluorescence spectra were recorded at room temperature.
4.6.3. Pure WPA5 for the MO Removal Capability Test
We further tested the ability of pure WPA5 to remove MO to verify the correctness of the MO removal scheme for WPA5-modified zeolites. The concentration of MO was held constant at 0.03 mM. Then, different equivalents of WPA5 were added (0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0, 2.2, 2.4, 2.6, 2.8, and 3.0 equiv). The UV spectral data were recorded after vortexing for 5 min and waiting for 1 h.
4.6.4. Docking Calculation
Furthermore, in order to clearly explain the formation mechanism of the MO/WP5A complex, molecular docking calculations were performed. Docking MO to the cavity of WP5A was based on the empirical free energy function and the Lamarck genetic algorithm. WP5A acted as a rigid acceptor molecule, while the MO acted as a ligand molecule allowing for flexible twisting. The WP5A was set to a grid box size to 5.0 nm × 5.0 nm × 5.0 nm; the grid spacing was 0.375 nm. Then, we calculated the grid points. The maximum evaluation number was 2.5 × 107, and the Lamarck genetic algorithm was used to perform 100 conformational searches. After the docking, the inclusion pattern of the MO/WP5A complex was discussed by cluster analysis. Molecular docking was performed on the AutoDock 4.2 program.45
Acknowledgments
This work was supported by NSFC (grant nos. 21762051, 21562048), Yunnan Provincial Department of Education Science Research Fund Project (grant no. 2019Y0193), and the Program for Innovative Research Team (in science and technology) at the University of Yunnan Province.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02180.
Synthetic of cationic water-soluble pillar[5]arene (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Herrera-González A. M.; Caldera V. M.; Peláez C. A. A. Adsorption of textile dyes using an activated carbon and crosslinked polyvinyl phosphonic acid composite. J. Environ. Manage. 2019, 234, 237–244. 10.1016/j.jenvman.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Satilmis B.; Uyar T. Amine modified electrospun PIM-1 ultrafine fibers for an efficient removal of methyl orange from an aqueous system. Appl. Surf. Sci. 2018, 453, 220–229. 10.1016/j.apsusc.2018.05.069. [DOI] [Google Scholar]
- Yoon S.; Bae S. Novel synthesis of nanoscale zerovalent iron from coal fly ash and its application in oxidative degradation of methyl orange by Fenton reaction. J. Hazard. Mater. 2019, 365, 751–758. 10.1016/j.jhazmat.2018.11.073. [DOI] [PubMed] [Google Scholar]
- Qiu B.; Xing M.; Zhang J. Mesoporous TiO2 nanocrystals grown in situ on graphene aerogels for high photocatalysis and lithium-ion batteries. J. Am. Chem. Soc. 2014, 136, 5852–5855. 10.1021/ja500873u. [DOI] [PubMed] [Google Scholar]
- Aldegs Y.; Elbarghouthi M.; Elsheikh A.; Walker G. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigm. 2008, 77, 16–23. 10.1016/j.dyepig.2007.03.001. [DOI] [Google Scholar]
- Sakir M.; Onses M. S. Solid substrates decorated with Ag nanostructures for the catalytic degradation of methyl orange. Results Phys. 2019, 12, 1133–1141. 10.1016/j.rinp.2018.12.084. [DOI] [Google Scholar]
- Haque E.; Jun J. W.; Jhung S. H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011, 185, 507–511. 10.1016/j.jhazmat.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Mahmoodian H.; Moradi O.; Shariatzadeha B.; Salehf T. A.; Tyagi I.; Maity A.; Asif M.; Gupta V. K. Enhanced removal of methyl orange from aqueous solutions by poly HEMA-chitosan-MWCNT nano-composite. J. Mol. Liq. 2015, 202, 189–198. 10.1016/j.molliq.2014.10.040. [DOI] [Google Scholar]
- Azami M.; Bahram M.; Nouri S.; Naseri A. Central composite design for the optimization of removal of the azo dye, methyl orange, from waste water using fenton reaction. J. Serb. Chem. Soc. 2012, 77, 235–246. 10.2298/jsc110315165a. [DOI] [Google Scholar]
- Gong R.; Ye J.; Dai W.; Yan X.; Hu J.; Hu X.; Li S.; Huang H. Adsorptive removal of methyl orange and methylene blue from aqueous solution with finger-citron-residue-based activated carbon. Ind. Eng. Chem. Res. 2013, 52, 14297–14303. 10.1021/ie402138w. [DOI] [Google Scholar]
- Li Y.; Liang F.; Bux H.; Yang W.; Caro J. Zeolitic imidazolate framework ZIF-7 based molecular sieve membrane for hydrogen separation. J. Membr. Sci. 2010, 354, 48–54. 10.1016/j.memsci.2010.02.074. [DOI] [Google Scholar]
- Gnanasekaran L.; Hemamalini R.; Saravanan R.; Ravichandran K.; Gracia F.; Agarwal S.; Gupta V. K. Synthesis and characterization of metal oxides (CeO2, CuO, NiO, Mn3O4, SnO2 and ZnO) nanoparticles as photocatalysts for degradation of textile dyes. J. Photochem. Photobiol., B 2017, 173, 43–49. 10.1016/j.jphotobiol.2017.05.027. [DOI] [PubMed] [Google Scholar]
- Ma H.; Wang B.; Luo X. Studies on degradation of Methyl Orange wastewater by combined electrochemical process. J. Hazard. Mater. 2007, 149, 492–498. 10.1016/j.jhazmat.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Darwish A. A. A.; Rashad M.; AL-Aoh H. A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dyes Pigm. 2019, 160, 563–571. 10.1016/j.dyepig.2018.08.045. [DOI] [Google Scholar]
- Gaffer A.; Al Kahlawy A. A.; Aman D. Magnetic zeolite-natural polymer composite for adsorption of chromium (VI). Egypt. J. Pet. 2017, 26, 995–999. 10.1016/j.ejpe.2016.12.001. [DOI] [Google Scholar]
- Habiba U.; Siddique T. A.; Joo T. C.; Salleh A.; Ang B. C.; Afifi A. M. Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium(VI) by flocculation/adsorption. Carbohydr. Polym. 2017, 157, 1568–1576. 10.1016/j.carbpol.2016.11.037. [DOI] [PubMed] [Google Scholar]
- Huang M.; Xu C.; Wu Z.; Huang Y.; Lin J.; Wu J. Photocatalytic discolorization of methyl orange solution by Pt modified TiO2 loaded on natural zeolite. Dyes Pigm. 2008, 77, 327–334. 10.1016/j.dyepig.2007.01.026. [DOI] [Google Scholar]
- Sabarish R.; Unnikrishnan G. Polyvinyl alcohol/carboxymethyl cellulose/ZSM-5 zeolite biocomposite membranes for dye adsorption applications. Carbohydr. Polym. 2018, 199, 129–140. 10.1016/j.carbpol.2018.06.123. [DOI] [PubMed] [Google Scholar]
- Mallard I.; Städe L. W.; Ruellan S.; Jacobsen P. A. L.; Larsen K. L.; Fourmentin S. Synthesis, characterization and sorption capacities toward organic pollutants of new β-cyclodextrin modified zeolite derivatives. Colloids Surf., A 2015, 482, 50–57. 10.1016/j.colsurfa.2015.04.014. [DOI] [Google Scholar]
- Kausar A.; Iqbal M.; Javed A.; Aftab K.; Nazli Z.-i. -H.; Bhatti H. N.; Nouren S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. 10.1016/j.molliq.2018.02.034. [DOI] [Google Scholar]
- Ogoshi T.; Kanai S.; Fujinami S.; Yamagishi T.-a.; Nakamoto Y. Para-bridged symmetrical pillar[5]arenes: their lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. 10.1021/ja711260m. [DOI] [PubMed] [Google Scholar]
- Fu H.-G.; Chen Y.; Liu Y. Multistimuli-Responsive and Photocontrolled Supramolecular Luminescent Gels Constructed by Anthracene-Bridged Bis(dibenzo-24-crown-8) with Secondary Ammonium Salt Polymer. ACS Appl. Mater. Interfaces 2019, 11, 16117–16122. 10.1021/acsami.9b04323. [DOI] [PubMed] [Google Scholar]
- Zhou W.-L.; Zhao X.; Chen Y.; Liu Y. Construction and heterogeneous photooxidization reactivity of a cyclodextrin/porphyrin polyrotaxane network. Org. Chem. Front. 2019, 6, 10–14. 10.1039/c8qo00790j. [DOI] [Google Scholar]
- Zhou W.; Chen Y.; Yu Q.; Li P.; Chen X.; Liu Y. Photo-responsive cyclodextrin/anthracene/Eu3+ supramolecular assembly for a tunable photochromic multicolor cell label and fluorescent ink. Chem. Sci. 2019, 10, 3346–3352. 10.1039/c9sc00026g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M.; Das M.; Savani C.; Thakore S.; Jadeja R. Maleic Anhydride Cross-Linked β-Cyclodextrin-Conjugated Magnetic Nanoadsorbent: An Ecofriendly Approach for Simultaneous Adsorption of Hydrophilic and Hydrophobic Dyes. ACS Omega 2019, 4, 11993–12003. 10.1021/acsomega.9b00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M.; Zhang Y.-M.; Liu Y. Selective binding affinity between quaternary ammonium cations and water-soluble calix[4]resorcinarene. J. Org. Chem. 2015, 80, 1849–1855. 10.1021/jo502825z. [DOI] [PubMed] [Google Scholar]
- Sharma V. S.; Shah A. P.; Sharma A. S. A new class of supramolecular liquid crystals derived from azo calix[4]arene functionalized 1,3,4-thiadiazole derivatives. New J. Chem. 2019, 43, 3556–3564. 10.1039/c8nj04997a. [DOI] [Google Scholar]
- Wu X.; Chen Y.; Yu Q.; Li F.-Q.; Liu Y. A cucurbituril/polysaccharide/carbazole ternary supramolecular assembly for targeted cell imaging. Chem. Commun. 2019, 55, 4343–4346. 10.1039/c9cc01601e. [DOI] [PubMed] [Google Scholar]
- Jie K.; Zhou Y.; Li E.; Huang F. Nonporous adaptive crystals of pillararenes. Acc. Chem. Res. 2018, 51, 2064–2072. 10.1021/acs.accounts.8b00255. [DOI] [PubMed] [Google Scholar]
- Tan L.-L.; Zhu Y.; Jin Y.; Zhang W.; Yang Y.-W. Highly CO2 selective pillar[n]arene-based supramolecular organic frameworks. Supramol. Chem. 2018, 30, 648–654. 10.1080/10610278.2018.1427239. [DOI] [Google Scholar]
- Tian H.; Wang C.; Li H.; Deng R.; Li R.; Meguellati K. A new cationic functionalized pillar[5]arene and applications for adsorption of anionic dyes. Eur. J. Org. Chem. 2019, 2019, 2819–2823. 10.1002/ejoc.201900256. [DOI] [Google Scholar]
- Wu X.; Chen Y.; Liu Y. Supramolecular crosslinked polymer for efficient organic dye removal from aqueous solution. Adv. Sustainable Syst. 2019, 3, 1800165–1800171. 10.1002/adsu.201800165. [DOI] [Google Scholar]
- Shi B.; Guan H.; Shangguan L.; Wang H.; Xia D.; Kong X.; Huang F. A pillar[5]arene-based 3D network polymer for rapid removal of organic micropollutants from water. J. Mater. Chem. A 2017, 5, 24217–24222. 10.1039/c7ta08894a. [DOI] [Google Scholar]
- Zhu H.; Wang H.; Shi B.; Shangguan L.; Tong W.; Yu G.; Mao Z.; Huang F. Supramolecular peptide constructed by molecular Lego allowing programmable self-assembly for photodynamic therapy. Nat. Commun. 2019, 10, 2412–2422. 10.1038/s41467-019-10385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.; Xu H.; Tong Z.; Yang Y.; Jiang G. Photo/pH-controlled host-guest interaction between an azobenzene-containing block copolymer and water-soluble pillar[6]arene as a strategy to construct the ″compound vesicles″ for controlled drug delivery. Mater. Sci. Eng. C 2018, 89, 237–244. 10.1016/j.msec.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Sun G.; He Z.; Hao M.; Zuo M.; Xu Z.; Hu X.-Y.; Zhu J.-J.; Wang L. Dual acid-responsive bola-type supramolecular vesicles for efficient intracellular anticancer drug delivery. J. Mater. Chem. B 2019, 7, 3944–3949. 10.1039/c9tb00555b. [DOI] [Google Scholar]
- Xiao T.; Zhong W.; Xu L.; Sun X.-Q.; Hu X.-Y.; Wang L. Supramolecular vesicles based on pillar[n]arenes: design, construction, and applications. Org. Biomol. Chem. 2019, 17, 1336–1350. 10.1039/c8ob03095b. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Chen X.; Ding J.; Yu L.; Ma D.; Ding J. Improved Solubility and bioactivity of camptothecin family antitumor drugs with supramolecular encapsulation by water-Soluble pillar[6]arene. ACS Omega 2017, 2, 5283–5288. 10.1021/acsomega.7b01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.-Y.; Gao L.; Mosel S.; Ehlers M.; Zellermann E.; Jiang H.; Knauer S. K.; Wang L.; Schmuck C. From Supramolecular Vesicles to Micelles: Controllable Construction of Tumor-Targeting Nanocarriers Based on Host-Guest Interaction between a Pillar[5]arene-Based Prodrug and a RGD-Sulfonate Guest. Small 2018, 14, 1803952–1803962. 10.1002/smll.201803952. [DOI] [PubMed] [Google Scholar]
- Wang X.; Wu J.-R.; Liang F.; Yang Y.-W. In situ gold nanoparticle synthesis mediated by a water-soluble leaning pillar[6]arene for self-assembly, detection, and catalysis. Org. Lett. 2019, 21, 5215–5218. 10.1021/acs.orglett.9b01827. [DOI] [PubMed] [Google Scholar]
- Ma X.-Q.; Wang Y.; Wei T.-B.; Qi L.-H.; Jiang X.-M.; Ding J.-D.; Zhu W.-B.; Yao H.; Zhang Y.-M.; Lin Q. A novel AIE chemosensor based on quinoline functionalized Pillar[5]arene for highly selective and sensitive sequential detection of toxic Hg2+ and CN–. Dyes Pigm. 2019, 164, 279–286. 10.1016/j.dyepig.2019.01.049. [DOI] [Google Scholar]
- Shamshoom C.; Fong D.; Li K.; Kardelis V.; Adronov A. Pillar[5]arene-Decorated Single-Walled Carbon Nanotubes. ACS Omega 2018, 3, 13935–13943. 10.1021/acsomega.8b02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-M.; He J.-X.; Zhu W.; Li Y.-F.; Fang H.; Yao H.; Wei T.-B.; Lin Q. Novel pillar[5]arene-based supramolecular organic framework gel for ultrasensitive response Fe3+ and F– in water. Mater. Sci. Eng. C 2019, 100, 62–69. 10.1016/j.msec.2019.02.094. [DOI] [PubMed] [Google Scholar]
- Hu X.-Y.; Ehlers M.; Wang T.; Zellermann E.; Mosel S.; Jiang H.; Ostwaldt J.-E.; Knauer S. K.; Wang L.; Schmuck C. Formation of Twisted b-Sheet Tapes from a Self-Complementary Peptide Based on Novel Pillararene-GCP Host–Guest Interaction with Gene Transfection Properties. Chem.—Eur. J. 2018, 24, 9754. 10.1002/chem.201801315. [DOI] [PubMed] [Google Scholar]
- Morris G. M.; Huey R.; Lindstrom W.; Sanner M. F.; Belew R. K.; Goodsell D. S.; Olson A. J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.