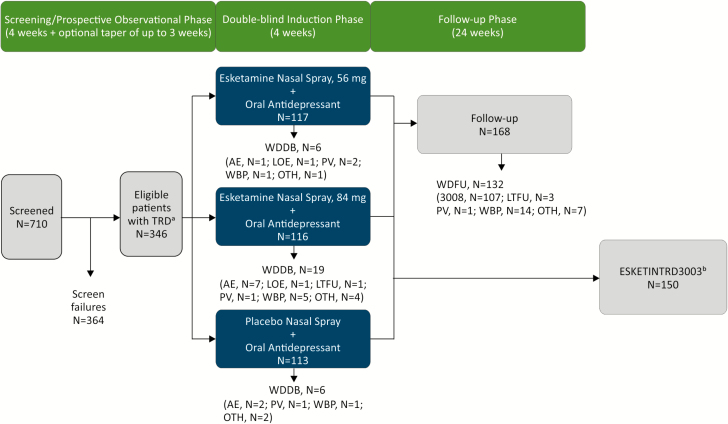
Figure 1.
Disposition of patients. AE, adverse event; LOE, lack of efficacy; LTFU, lost to follow-up; MADRS, Montgomery-Asberg Depression Rating Scale; OTH, other reason for withdrawal; PV, protocol violation; TRD, treatment-resistant depression; WBP, withdrawal by patient; WDDB, withdrawal from double-blind phase; WDFU, withdrawn from follow-up phase. 3008 entered Janssen-sponsored Study TRD3008 (NCT02782104). aPatients with nonresponse to ≥2 oral antidepressants, 1 observed prospectively, prior to randomization were eligible to participate in the study. bResponders (defined as ≥50% reduction in MADRS total score from baseline to end of the 28-day double-blind phase) were eligible to continue to Janssen-sponsored Study ESKETINTRD3003 (NCT02493868).