Figure 1. Outline of the experimental design.
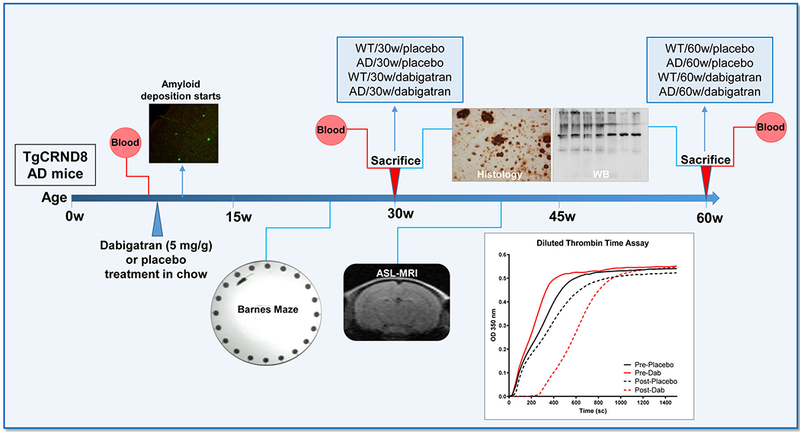
TgCRND8 AD mice and WT littermates (n=13-18 female mice/group) started dabigatran/placebo treatment at 8 weeks-of-age, and were sacrificed after 22 weeks (at 30-week-old, n=8-11 mice/group) or 52 weeks of treatment (at 60-week-old, n=5-7 mice/group). Histology and WB assays were performed in the 8 experimental groups (WT/30w/placebo; AD/30w/placebo; WT/30w/dabigatran; AD/30w/dabigatran; WT/60w/placebo; AD/60w/placebo; WT/60w/dabigatran and AD/60w/dabigatran). Blood was extracted before and after treatment to measure dabigatran concentration by diluted thrombin time assays (representative graph is shown). Barnes maze to assess spatial memory performance was performed 17 weeks after the treatment started (at 25-week-old, n=12-16 mice/group). Cerebral perfusion was measured non-invasively by ASL-MRI 32 weeks after treatment started (at 40-week-old, n=5-7 mice/group). AD=Alzheimer’s disease; ASL=Arterial Spin Labeling, MRI=Magnetic Resonance Imaging, WT=wild type, WB=western blot.
