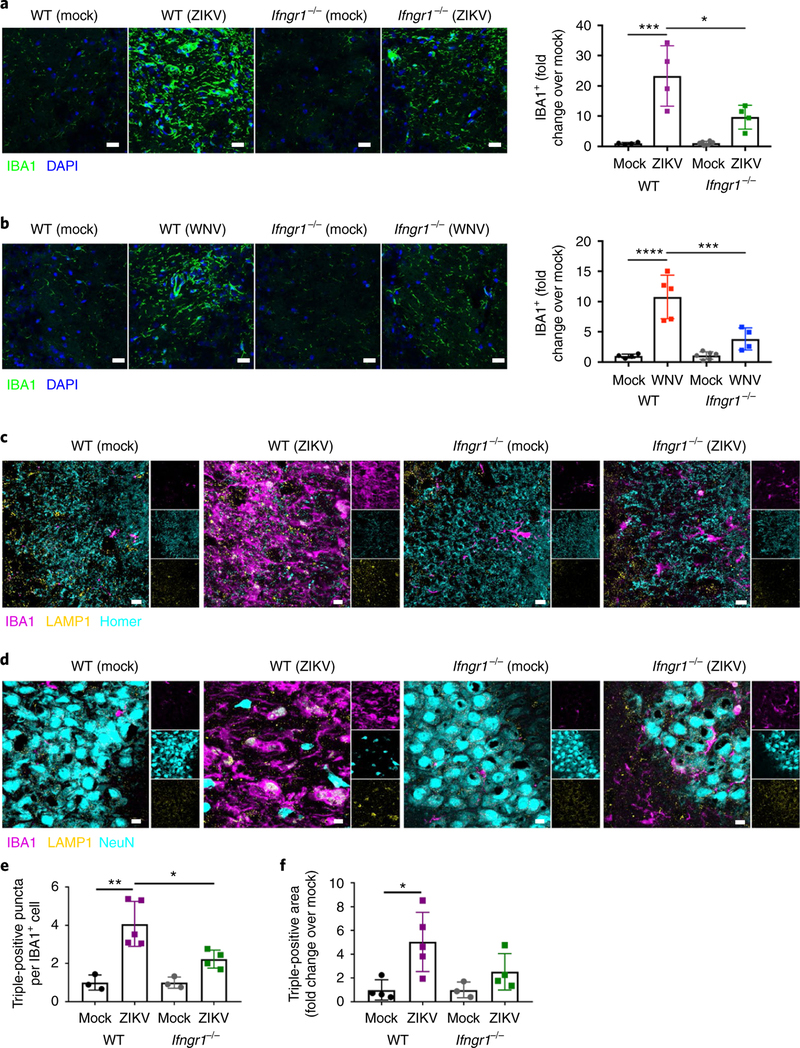
Fig. 7 |. IFN-γ potentiates microglial engulfment of postsynaptic termini and neurons in ZiKV-infected animals post recovery.
a,b, WNV or ZIKV infection induces increased expression of IBA1 in the hippocampus at 25d.p.i., which is significantly reduced in Ifngrl−/− mice. Representative immunostaining (left) and quantitation (right) of the hippocampus at 25d.p.i. Data were pooled from two independent experiments. For a, n = 4 mice per group; P=0.0001 (left) and P = 0.0102 (right). For b, n = 4 (WT mock, WT WNV, Ifngrl−/− WNV) or 6 (Ifngrl−/− mock) mice per group; P< 0.0001 (left) and P = 0.0010 (right). Scale bars, 50 μm. c,e, IBA1+ microglia engulf postsynaptic termini at 25d.p.i., which is significantly reduced in Ifngrl−/− mice. Representative immunostaining, with single-channel images on the right (c) and quantitation (e) of the hippocampus of infected mice post recovery. Data were pooled from two independent experiments (n = 3 (WT Mock, Ifngrl−/− Mock), 5 (WT ZIKV), or 4 (Ifngrl−/− ZIKV) mice per group; P= 0.0011 (left) and P = 0.0209 (right)). Scale bars, 10 μm. d,f, IBA1+ microglia engulf NeuN+ perikarya at 25d.p.i., which is reduced in Ifngrl−/− mice. Representative immunostaining, with single-channel images on the right (d) and quantitation (f) of the hippocampus of infected mice post recovery. Data were pooled from two independent experiments (n = 4 (WT Mock, Ifngrl−/− ZIKV), 5 (n = WT ZIKV), or 3 (Ifngrl−/− Mock) mice per group; P = 0.0190). Scale bars, 10μm. For a, b, e, and f, data represent the mean±s.d. and were analyzed by two-way ANOVA, corrected for multiple comparisons. *P<0.05, ***P< 0.001, ****P< 0.0001.