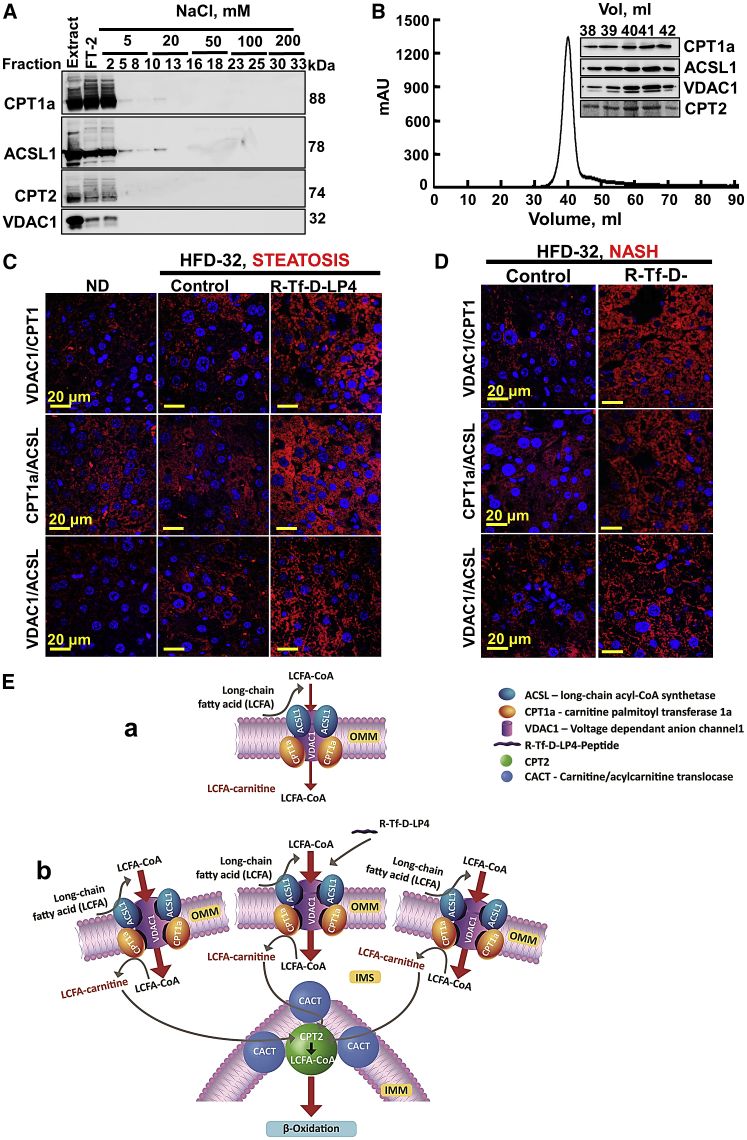
Figure 9.
Purification of a VDAC1/CPT1a/ACSL1 Complex and Peptide Treatment of HFD-32-Fed Mice Increase Complex Levels and Association
(A) Immunoblot of complexed VDAC1, CPT1a, ACSL1, and CPT2 isolated from rat liver mitochondria using reactive red agarose (RRA). (B) Sephacryl S-200 chromatography with absorbance at 280 nm (presented as mAU) of elution fractions is shown, and the inset shows immunoblots of the peak fractions as functions of the elution volume (Vol). (C and D) Liver sections from HFD-32-fed mice untreated and peptide treated (14 mg/kg) at the steatosis (C) or NASH (D) stage were subjected to in situ PLA to test for interactions between VDAC1 and CPT1a or CPT1a and ACSL1 or VDAC1 and ACSL1 (see the Supplemental Materials and Methods). DAPI-stained nuclei are shown. (E) Schematic presentation of proposed mode of peptide-mediated increased β-oxidation in steatosis and NASH, as induced by an HFD-32 diet. The complex ACSL1/VDAC1/CPT1a and CPT2 is modified by the VDAC1-derived peptide. (a) VDAC1 serves as an anchoring site for ACSL1, associated with the outer surface of the OMM, and CPT1a, which faces the IMS. The acyl-CoA formed by ACSL1 is transferred across the OMM by VDAC1 to the IMS, where CPT1a converts it into acyl-carnitine that is transported by CACT into the matrix, where it is converted back into acyl-CoA via IMM-associated CPT2 and, subsequently, undergoes β-oxidation in the matrix. (b) The VDAC1-based peptide serves as a decoy, interacting with CPT1a and/or ACSL1 such that their interaction with VDAC1 is modified, leading to increased fatty acid translocation to the mitochondria, thereby increasing β-oxidation. The presence of CPT2 in the purified complex suggests the direct channeling of fatty acids from the cytosol to the matrix for β-oxidation.