Abstract
Photocatalytic hydrogen production from water splitting is of auspicious possibility to resolve the energy shortage and environmental anxieties. In the past decade, the combination of different carbon-based allotropes with semiconductors of different structure and unique properties to construct heterojunction, which can improve the charge separation, light absorption, and steadiness, offer a promising way to achieve efficient photocatalyst. This review aims to provide an overview of the development for the carbon nanomaterials (CNMs)-based photocatalysts used for hydrogen production from water splitting and photocatalytic degradation of organic pollutants in waste water. The recent progress of CNMs-based heterojunction, including various composite with graphene, fullerene, carbon quantum dots (CQDs), and carbon nanotubes (CNTs) were highlighted. Furthermore, a typical model of CNMs-based Z-scheme heterojunction was also addressed. Finally, a promising perspective on the future development of CNMs-based photocatalysts have been discussed.
Keywords: carbon nanomaterials (CNMs), photocatalysts, heterojunction, water splitting, hydrogen production
Introduction
Photocatalysts facing toward energy crisis and environmental issues have attracted increased intention as one of the best way for the reduction of toxic contaminants and H2 production (Hisatomi et al., 2014; Low et al., 2015; Dai et al., 2017; Liu G. et al., 2019). However, challenges for the photocatalysts remains regarding to the limited light absorption, high charge recombination, and low quantum yield (Sudhaik et al., 2018). Up to now various photocatalysts have been developed to resolve these issues, among which carbon-based photocatalysts recently aroused tremendous interest due to their large surface area, favorable electronic conductivity, low fabrication cost, and high chemical/thermal stability (Yang et al., 2014; Xia et al., 2017; Ma et al., 2018). These unique properties make carbon nanomaterials (CNMs) as the most promising candidate for photocatalysts (Yu et al., 2014).
The most widely used CNMs for the synthesis of photocatalysts, such as graphene (Yu et al., 2016), carbon nanotubes (CNTs) (Zhang Y. et al., 2019), carbon quantum dots (CQDs) (Li Y. et al., 2018), fullerene (Song et al., 2017), and graphitic carbon nitride (g-C3N4) (Zhang S. et al., 2019) have attracted great attention due to their high physiochemical stability, earth abundant, and low synthesis cost. Moreover, the electronic structure and photocatalytic properties of CNMs could be adjusted through morphology and interfacial modulation (Xin et al., 2018). Whereas, pristine CNMs suffer from rapid recombination of electron-hole pair and narrow visible light adsorption. One of the best strategy to solve this problem is to construct heterojunction via assembly of CNMs with semiconductors. Especially, the modified CNMs-based Z-scheme heterojunction, resembling the natural photosynthetic model, benefit from various merits including improved light harvesting, spatially separated electron and hole sites and strong redox ability (Tong et al., 2012). Beside the structure modification of the Z-scheme heterojunction, the introduced CNMs also serve as electron mediator between two semiconductors, which actually reduce the resistance and improve the charge separation and stability.
This paper aims to provide an overview of carbon-based photocatalysts in water splitting for H2 production as well as degradation of organic pollutants. The properties, performances, and combinations of different allotropes of carbon as photocatalysts were discussed. Photocatalytic enhancements by solid Z-scheme heterojunction were also reviewed.
Carbon-Based Photocatalysts
Graphene as Photocatalyst
Graphene with excellent physical and chemical properties discovered in 2004, holding sp2-hybridized atoms tightly assembled into an ordered two-dimensional (2D) honeycomb construct, offer new opportunities in designing efficient photocatalytic materials with high stability (Gupta et al., 2019; Madkour, 2019). Recent demand for the synthesis of metal-free photocatalysts is on the verge of increase. Gong et al. successfully obtained graphene/g-C3N4 nanocomposites by impregnation chemical reduction strategy, which served as active photocatalysts for H2 production in visible light (Gong et al., 2018). Moreover, 2D graphene and transition metal dichalcogenides (TMDCs), have become versatile nanomaterials for the fast progress of photocatalysts due to their unique properties in optical, electrical, thermal, and mechanical aspects (Rosman et al., 2018). One of the works done by Lv et al. that elaborated graphene composite without noble metal, revealed that graphene attached to semiconductor surface fabricated by hydrothermal method can efficiently accommodate and transport electrons from the excited semiconductor, which not only hindered charge recombination but also improved charge transfer, giving rise to high photocatalytic efficiency (Lv et al., 2012). This work confirmed the significant contribution of graphene in enhancing the photocatalytic activity. Afterwards, many graphene-based photocatalysts have been developed. For example, Quiroz-Cardoso et al. (2019) recently reported graphene in combination with nickel nanoparticles modified CdS fibers (Ni/GO-CdS) enhanced the photocatalytic hydrogen production, which was 6.3 times higher than that of bare CdS. Considering the superior conductivity and tunable structure, graphene would be the most promising candidate for photocatalysts. Design and construction of novel hierarchical architectures hybridizing with graphene nanostructures would provide plenty of rooms for photocatalytic application.
CNTs as Photocatalyst
Photocatalytic water-splitting technology based on CNTs-modified nanomaterials has exhibited great potential for hydrogen production in view of their low cost and high stability (Yi et al., 2018). For example, Zheng et al. (2008) has offered new opportunities for achieving high photocatalytic activity with high stability. In combination of CNTs with graphene not only increase reaction sites but also inhibit the recombination of photo-excited electron-hole pairs (Bhanvase et al., 2017). In addition, CNTs-based photocatalysts also revealed high activity on the photocatalytic degradation of organic pollutants due to π-system or formation of heterojunction. For example, CNT-modified hierarchical microspheres ZnO enhanced the visible light adsorption and charge separation process, exhibiting excellent photocatalytic performance much better than the pure ZnO for the reduction of the organic molecules in the industrial effluents (Ahmad et al., 2014). Combination of CNT with other photocatalysts could enhance the conductivity and facilitate the charge transfer process during the photocatalytic reaction. To further improve the performance in future, more efforts should be made to in-situ synthesize CNT-based composite in order to strengthen the synergetic interaction between CNT and other nanostructures.
CQDs as Photocatalyst
CQDs as an emerging and recently developed CNMs provided well-controlled intrinsic characteristics because of its unique optical and electrical properties, as well as the special fluorescence emission feature (Zhang et al., 2017). Since their discovery in 2004 (Xu et al., 2004), CQDs have been utilized in various application, including chemical sensor, bioimaging, nanomedicine, photocatalysts, etc. Particularly, in photocatalytic application, CQDs showed the most promising potential for photocatalytic H2 production. Moreover, CQDs can act both as electron acceptor and donor leading to effective electron and hole separation, and extensively modify the photo-absorption range of semiconductor materials with large band gap to visible regions (Pirsaheb et al., 2018). Wang et al. demonstrated that metal-doped CQDs combined with CdS nanowires as a co-catalyst showed much better hydrogen production performance than the undoped CQDs/CdS composite (Wang Y. et al., 2019). One more example examined by Wang et al. demonstrated that the visible-light-sensitive BiVO4 quantum tube (q-BiVO4) decorated with CQDs displayed outstanding photocatalytic performance, whose kinetic constants for the degradation of phenol and rhodamine B (RhB) were 3.0 and 2.4 times higher than those of the sole q-BiVO4, respectively (Wang G. et al., 2019). Due to the potential both as electron donor and acceptor, CQD should be further investigated in the field of photocatalytic application. Developing novel and facile green synthesis method to fabricate CQD-based CNMs, especially the metal-free catalysts deserves more attention.
Fullerene as Photocatalyst
Fullerene (C60) with a close-shell shape consisting of 20 hexagons and 12 pentagons, holding 30 orbital bonding with 60 p-electrons, has been recognized as the most significant carbon allotropes because of the unique chemical and physical characteristics (Lindqvist et al., 2014). Besides, C60 is both an excellent electron acceptor and donor, which facilitate the functionality of fullerene-based carbon materials in photocatalytic applications. Encapsulation of fullerene into CNTs is super effective technique, pioneered by Smith et al. (1998), to fabricate heterojunction with unique electronic characteristics (Rahimi-Nasrabadi et al., 2017). Song et al. synthesized a novel C60/graphene/g-C3N4 composite with high hydrogen production efficiency for water splitting (Song et al., 2017). The synergetic effect between graphene and C60 improved the transportation and utilization efficiency of photo-generated electrons and accelerated the separation of photo-generated electron and hole pairs, thus considerably enhancing the hydrogen generation ability of g-C3N4. Fullerene and its derivatives have been widely used in the organic photovoltaic device, however, their application in photocatalysis for hydrogen production and organic pollutants degradation is in the infancy. Until now, most works only focused on the C60, other members in fullerene family such as C70 and their derivatives should be pay more attention for photocatalysts in future.
g-C3N4 as Photocatalyst
Recently, the improvement of photocatalytic activity by using g-C3N4 has turned into a hot research subject because of its tunable electronic band structure, highly stable physiochemical properties, simple manufacturing and low cost (Dong et al., 2016, 2019; Li Y. et al., 2018). Wang J. et al. (2019) fabricated a 3D flower-like TiO2 hybridized with 2D g-C3N4 nanosheet through a hydrothermal and calcination process. The resulting TiO2/g-C3N4 composite exhibited a much enhanced efficiency of photocatalytic hydrogen production, which is 7.7 and 1.9 times higher than that of the pure g-C3N4 and TiO2, respectively. It was reported that the extended visible light adsorption by g-C3N4 make a contribution to the improved photocatalytic performance. In addition, the activity of g-C3N4 could also be improved by doping. Zhou et al. (2019) reported that the NO removal rate of g-C3N4 could be enhanced by 1.5 times after Sr doping. Density functional theory (DFT) method is powerful for systematically depicting the electronic structures and understanding energy-related mechanism for photocatalytic reaction. The results revealed that different doping modes of Sr including intercalation, cavity padding, replacement of triazine N and bridging N could decrease the band gap of g-C3N4, thus facilitating the charge transfer process.
Heterojunction
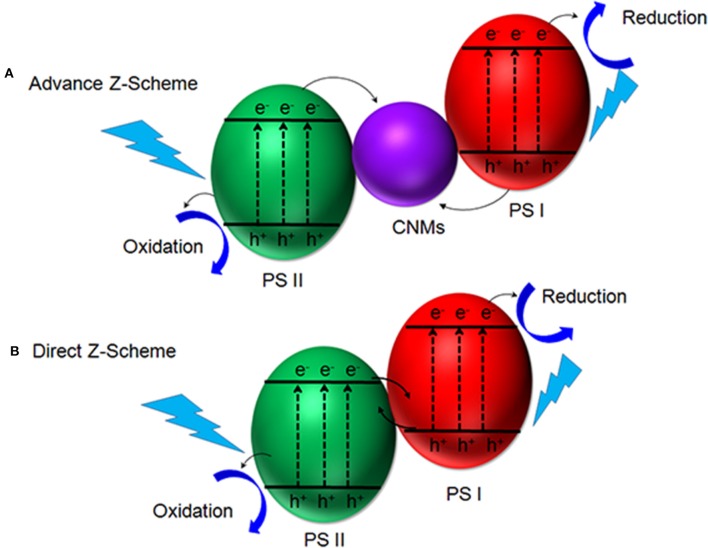
Many efforts have been made to realize the complete utilization of photo-excited charge carriers and inhibit recombination of electron-hole pairs during the photocatalytic process, among which fabrication of heterojunction is one of the best approach to improve the charge separation efficiency and reduce the recombination of the photogenerated electron-hole pairs (Moniz et al., 2015; Wang et al., 2018). The most commonly-investigated heterojunction is constructed via two solid semiconductors and one electron mediator as illustrated in Figure 1, forming an advanced solid-type Z-scheme configuration. The main mechanism for Z-scheme heterojunction is inspired by the natural photosynthesis process. Electrons of photocatalysts I are recombined with holes in photocatalysts II via electron mediator under light, which inhibits the recombination of photo-induced charge carrier and stay their redox property (Bard and Fox, 1995).
Figure 1.
Schematic illustrations of an advanced Z-scheme heterojunction with CNMs as an electron mediator (A) and the direct Z-scheme heterojunction (B). Modified from Li H. et al. (2015). with copyright permission from John Wiley and Sons, Inc.
In order to improve the conductivity of semiconductor photocatalysts, CNMs like graphene, fullerene, CNTs and their derivatives have been widely used in different heterojunction as an electron mediator to increase the conductivity (Natarajan et al., 2018). Table 1 summarize several typical carbon-based photocatalysts. As compared with other carbon-based heterojunction photocatalysts, graphene exhibits a plenty of merits such as low cost, large surface area and tunable band structure (Li X. et al., 2018). Gebreslassie et al. demonstrated that graphene as an electron mediator can significantly enhance the photocatalytic activity, which showed 7–15-folds higher H2 production compared with their pristine compounds without the aid of graphene (Gebreslassie et al., 2019). Similarly, Jiang et al. synthesized a solid-state Z-scheme Bi2WO6/CNTs/g-C3N4 composite, where CNT acted as an electron mediator (Jiang et al., 2018). This composite disclosed an outperforming photocatalytic activity than the pure Bi2WO6 and g-C3N4 for the degradation of 2,4-dibromophenol. Recently, CQDs have also been used as electron mediator to build solid-state Z-scheme heterojunction. In 2019, Liu et al. fabricated a CQD-based Z-scheme heterojunction by bridging TiO2 and Cd0.5Zn0.5S with CQDs, which exhibits super photocatalytic activity for H2 evolution (Liu E. et al., 2019). Meanwhile, Pan et al. constructed a sandwich-type structure, where CQDs were embedded between CdS and BiOCl (Pan et al., 2018). The resulting CdS/CQDs/BiOCl heterojunction displayed much higher photocatalytic activity on the degradation of RhB and phenol under visible and UV light illumination compared with BiOCl, CdS/BiOCl, and CQDs/BiOCl.
Table 1.
Summary of carbon-based photocatalysts.
| Photocatalysts | Heterojunction type | Synthesis method | References |
|---|---|---|---|
| PPTA/MWNTs | N.A.a | Polycondensation | Mazrouaa et al., 2019 |
| g-C3N4/graphene/NiFe2O4 | Solid state Z-scheme | Hydrothermal | Gebreslassie et al., 2019 |
| CN/CNT/BWO | Solid state Z-scheme | N.A. | Jiang et al., 2018 |
| Bi2WO6/g-C3N4 | Direct Z-scheme | Hydrothermal | Li M. et al., 2015 |
| ZnO/g-C3N4 | Direct Z-scheme | Solid state | Yu et al., 2015 |
| Cd0.5Zn0.5S/CQD/TiO2 | Solid state Z-scheme | Hydrothermal | Liu E. et al., 2019 |
| Cds/CQDs/BiOCl | Solid state Z-scheme | Facile-region | Pan et al., 2018 |
| Ru/SrTiO3 | Z-scheme | Hummers method | Iwase et al., 2011 |
| SnS2/g-C3N4 | Z-scheme | Hydrothermal | Di et al., 2017 |
| SnO2−x/g-C3N4 | Z-scheme | Solid-state synthesis | He et al., 2015 |
| CdS/SiC | Z-scheme | Hydrothermal | Peng et al., 2015 |
| CdS/graphene | N.A. | N.A. | Li et al., 2011 |
| ZnIn2S4/RGO | N.A. | Solvothermal | Ye et al., 2014 |
| Bi2WO6/graphene | N.A. | Sonochemical | Sun et al., 2014 |
| Graphene/g-C3N4 | N.A. | Impregnation–chemical reduction | Xiang et al., 2011 |
| Nanoparticle/graphene | N.A. | One-pot solution | Lv et al., 2012 |
| TiO2/graphene | N.A. | Sol gel method | Zhang et al., 2010 |
| TiO2/carbon dots | N.A. | Hydrothermal | Wang et al., 2014 |
| CdS/graphene | N.A. | Hydrothermal | Ye et al., 2012 |
| Ta2O5/CNT | Schottky heterojunction | N.A. | Cherevan et al., 2014 |
| Ni/GO-CdS | N.A. | Photo-deposition | Quiroz-Cardoso et al., 2019 |
| La-CNTs/TiO2 | N.A. | Sol-gel method | Tahir, 2019 |
| TiO2/CQD | N.A. | Green synthesis | Sargin et al., 2019 |
N.A., Not Available.
Conclusions and Perspectives
Carbon-based nanomaterials with low cost and favorable catalytic performance have been extensively used for photocatalytic reactions in the fields of energy conversion and environmental protection. In this review, CNMs such as graphene, CNTs, CQDs, C60, and g-C3N4, etc. used as photocatalysts in the application for H2 production from water splitting and photocatalytic degradation of organic pollutants in waste water were comprehensively overviewed. It could be concluded that CNMs exhibit intriguing property in enhancing the photocatalytic performance of various photocatalysts. With the rapid development of advanced technique, various carbon-based Z-scheme heterojunction with excellent photocatalytic performance have been established. This type of heterojunction inspired by artificial photosynthesis method possess many advantages including increased light harvesting and favorable strong redox capability, which highly improved the photocatalytic performance compared with the direct heterojunction. Different type of carbon allotropes are the good performer as an electron mediator in solid-state Z-scheme heterojunction while the selection of proper electron mediator with specific composite to different materials according to their specific function is challenging and crucial. Obviously photocatalytic efficiency depends on the type of material. The development of novel photocatalysts with better catalytic performance has always been put forward to the frontiers of nanomaterials and a further understanding of heterojunction mechanisms are also of great importance to promote the application of photocatalysts.
Author Contributions
NS and YF organized and wrote the manuscript. XW, JH, and LC discussed the results and revised the paper. All authors approved this publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer DG declared a shared affiliation, with no collaboration, with the authors NS, JH, YF, XW, LC, to the handling editor at time of review.
Footnotes
Funding. This work was financially supported by the National Natural Science Foundation of China (21603243), Beijing National Laboratory for Molecular Sciences (BNLMS201805), Natural Science Foundation of Shaanxi Province (2019JQ-203), Key Laboratory of Auxiliary Chemistry and Technology for Chemical Industry (KFKT2019-06), and Natural Science Foundation of Shaanxi Provincial Department of Education (17JK0093). YF was grateful for the support from the 1000 Youth Talents Plan of Shaanxi Province, Platform Construction Fund for Imported Talent of Shaanxi University of Science and Technology (134080038) and Youth Talents of Shaanxi University of Science and Technology (2016QNBJ-14).
References
- Ahmad M., Ahmed E., Hong Z. L., Ahmed W., Elhissi A., Khalid N. R. (2014). Photocatalytic, sonocatalytic and sonophotocatalytic degradation of Rhodamine B using ZnO/CNTs composites photocatalysts. Ultrason. Sonochem. 21, 761–773. 10.1016/j.ultsonch.2013.08.014 [DOI] [PubMed] [Google Scholar]
- Bard A. J., Fox M. A. (1995). Artificial photosynthesis: solar splitting of water to hydrogen and oxygen. Acc. Chem. Res. 28, 141–145. 10.1021/ar00051a007 [DOI] [Google Scholar]
- Bhanvase B. A., Shende T. P., Sonawane S. H. (2017). A review on graphene–TiO2 and doped graphene–TiO2 nanocomposite photocatalyst for water and wastewater treatment. Environ. Tech. Rev. 6, 1–14. 10.1080/21622515.2016.1264489 [DOI] [Google Scholar]
- Cherevan A. S., Gebhardt P., Shearer C. J., Matsukawa M., Domen K., Eder D. (2014). Interface engineering in nanocarbon–Ta2O5 hybrid photocatalysts. Energ. Environ. Sci. 7, 791–796. 10.1039/C3EE42558D [DOI] [Google Scholar]
- Dai D., Xu H., Ge L., Han C., Gao Y., Li S., et al. (2017). In-situ synthesis of CoP co-catalyst decorated Zn0.5Cd0.5S photocatalysts with enhanced photocatalytic hydrogen production activity under visible light irradiation. Appl. Catal. B Environ. 217, 429–436. 10.1016/j.apcatb.2017.06.014 [DOI] [Google Scholar]
- Di T., Zhu B., Cheng B., Yu J., Xu J. (2017). A direct Z-scheme g-C3N4/SnS2 photocatalyst with superior visible-light CO2 reduction performance. J. Catal. 352, 532–541. 10.1016/j.jcat.2017.06.006 [DOI] [Google Scholar]
- Dong G., Yang L., Wang F., Zang L., Wang C. (2016). Removal of nitric oxide through visible light photocatalysis by g-C3N4 modified with perylene imides. ACS Catal. 6, 6511–6519. 10.1021/acscatal.6b01657 [DOI] [Google Scholar]
- Dong G., Zhao L., Wu X., Zhu M., Wang F. (2019). Photocatalysis removing of NO based on modified carbon nitride: the effect of celestite mineral particles. Appl. Catal. B Environ. 245, 459–468. 10.1016/j.apcatb.2019.01.013 [DOI] [Google Scholar]
- Gebreslassie G., Bharali P., Chandra U., Sergawie A., Boruah P. K., Das M. R., et al. (2019). Novel g-C3N4/graphene/NiFe2O4 nanocomposites as magnetically separable visible light driven photocatalysts. J. Photoch. Photobio. A 382:111960 10.1016/j.jphotochem.2019.111960 [DOI] [Google Scholar]
- Gong S., Jiang Z., Zhu S., Fan J., Xu Q., Min Y. (2018). The synthesis of graphene-TiO2/g-C3N4 super-thin heterojunctions with enhanced visible-light photocatalytic activities. J. Nanopart. Res. 20:310 10.1007/s11051-018-4399-8 [DOI] [Google Scholar]
- Gupta N., Rai D. B., Jangid A. K., Kulhari H. (2019). A review of theranostics applications and toxicities of carbon nanomaterials. Curr. Drug Metab. 20, 506–532. 10.2174/1389200219666180925094515 [DOI] [PubMed] [Google Scholar]
- He Y., Zhang L., Fan M., Wang X., Walbridge M. L., Nong Q., et al. (2015). Z-scheme SnO2-x/g-C3N4 composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction. Sol. Energ. Mater. Sol. C. 137, 175–184. 10.1016/j.solmat.2015.01.037 [DOI] [Google Scholar]
- Hisatomi T., Kubota J., Domen K. (2014). Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 43, 7520–7535. 10.1039/C3CS60378D [DOI] [PubMed] [Google Scholar]
- Iwase A., Ng Y. H., Ishiguro Y., Kudo A., Amal R. (2011). Reduced graphene oxide as a solid-state electron mediator in z-scheme photocatalytic water splitting under visible light. J. Am. Chem. Soc. 133, 11054–11057. 10.1021/ja203296z [DOI] [PubMed] [Google Scholar]
- Jiang D., Ma W., Xiao P., Shao L., Li D., Chen M. (2018). Enhanced photocatalytic activity of graphitic carbon nitride/carbon nanotube/Bi2WO6 ternary Z-scheme heterojunction with carbon nanotube as efficient electron mediator. J. Colloid Interf. Sci. 512, 693–700. 10.1016/j.jcis.2017.10.074 [DOI] [PubMed] [Google Scholar]
- Li H., Zhou Y., Tu W., Ye J., Zou Z. (2015). State-of-the-art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Adv. Fun. Mater. 25, 998–1013. 10.1002/adfm.201401636 [DOI] [Google Scholar]
- Li M., Zhang L., Fan X., Zhou Y., Wu M., Shi J. (2015). Highly selective CO2 photoreduction to CO over g-C3N4/Bi2WO6 composites under visible light. J. Mater. Chem. A 3, 5189–5196. 10.1039/C4TA06295G [DOI] [Google Scholar]
- Li Q., Guo B., Yu J., Ran J., Zhang B., Yan H., et al. (2011). Highly efficient visible-light-driven photocatalytic hydrogen production of CdS-cluster-decorated graphene nanosheets. J. Am. Chem. Soc. 133, 10878–10884. 10.1021/ja2025454 [DOI] [PubMed] [Google Scholar]
- Li X., Shen R., Ma S., Chen X., Xie J. (2018). Graphene-based heterojunction photocatalysts. Appl. Surf. Sci. 430, 53–107. 10.1016/j.apsusc.2017.08.194 [DOI] [Google Scholar]
- Li Y., Feng X., Lu Z., Yin H., Liu F., Xiang Q. (2018). Enhanced photocatalytic H2-production activity of C-dots modified g-C3N4/TiO2 nanosheets composites. J. Colloid Interf. Sci. 513, 866–876. 10.1016/j.jcis.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Lindqvist C., Bergqvist J., Feng C.-C., Gustafsson S., Bäcke O., Treat N. D., et al. (2014). Fullerene nucleating agents: a route towards thermally stable photovoltaic blends. Adv. Energ. Mater. 4:1301437 10.1002/aenm.201301437 [DOI] [Google Scholar]
- Liu E., Xu C., Jin C., Fan J., Hu X. (2019). Carbon quantum dots bridged TiO2 and Cd0.5Zn0.5S film as solid-state Z-scheme photocatalyst with enhanced H2 evolution activity. J. Taiwan Inst. Chem. Eng. 97, 316–325. 10.1016/j.jtice.2019.02.027 [DOI] [Google Scholar]
- Liu G., Wang G., Hu Z., Su Y., Zhao L. (2019). Ag2O nanoparticles decorated TiO2 nanofibers as a pn heterojunction for enhanced photocatalytic decomposition of RhB under visible light irradiation. Appl. Surf. Sci. 465, 902–910. 10.1016/j.apsusc.2018.09.216 [DOI] [Google Scholar]
- Low J., Yu J., Ho W. (2015). Graphene-based photocatalysts for CO2 reduction to solar fuel. J. Phys. Chem. Lett. 6, 4244–4251. 10.1021/acs.jpclett.5b01610 [DOI] [PubMed] [Google Scholar]
- Lv X.-J., Fu W.-F., Chang H.-X., Zhang H., Cheng J.-S., Zhang G.-J., et al. (2012). Hydrogen evolution from water using semiconductor nanoparticle/graphene composite photocatalysts without noble metals. J. Mater. Chem. 22, 1539–1546. 10.1039/C1JM14502A [DOI] [Google Scholar]
- Ma X., Xiang Q., Liao Y., Wen T., Zhang H. (2018). Visible-light-driven CdSe quantum dots/graphene/TiO2 nanosheets composite with excellent photocatalytic activity for E. coli disinfection and organic pollutant degradation. Appl. Surf. Sci. 457, 846–855. 10.1016/j.apsusc.2018.07.003 [DOI] [Google Scholar]
- Madkour L. H. (2019). Carbon Nanomaterials and Two-Dimensional Transition Metal Dichalcogenides (2D TMDCs). Nanoelectronic Materials: Fundamentals and Applications. Cham: Springer, 165–245. [Google Scholar]
- Mazrouaa A. M., Mansour N. A., Abed M. Y., Youssif M. A., Shenashen M. A., Awual M. R. (2019). Nano-composite multi-wall carbon nanotubes using poly(p-phenylene terephthalamide) for enhanced electric conductivity. J. Environ. Chem. Eng. 7:103002 10.1016/j.jece.2019.103002 [DOI] [Google Scholar]
- Moniz S. J. A., Shevlin S. A., Martin D. J., Guo Z.-X., Tang J. (2015). Visible-light driven heterojunction photocatalysts for water splitting – a critical review. Energ. Environ. Sci. 8, 731–759. 10.1039/C4EE03271C [DOI] [Google Scholar]
- Natarajan T. S., Thampi K. R., Tayade R. J. (2018). Visible light driven redox-mediator-free dual semiconductor photocatalytic systems for pollutant degradation and the ambiguity in applying Z-scheme concept. Appl. Catal. B Environ. 227, 296–311. 10.1016/j.apcatb.2018.01.015 [DOI] [Google Scholar]
- Pan J., Liu J., Zuo S., Khan U. A., Yu Y., Li B. (2018). Structure of Z-scheme CdS/CQDs/BiOCl heterojunction with enhanced photocatalytic activity for environmental pollutant elimination. Appl. Surf. Sci. 444, 177–186. 10.1016/j.apsusc.2018.01.189 [DOI] [Google Scholar]
- Peng Y., Guo Z., Wang D., Pan N., Yuan W. (2015). Heterogeneous nucleation of CdS to enhance visible-light photocatalytic hydrogen evolution of SiC/CdS composite. Appl. Phys. Lett. 107:012102 10.1063/1.4923399 [DOI] [Google Scholar]
- Pirsaheb M., Asadi A., Sillanpää M., Farhadian N. (2018). Application of carbon quantum dots to increase the activity of conventional photocatalysts: A systematic review. J. Mol. Liq. 271, 857–871. 10.1016/j.molliq.2018.09.064 [DOI] [Google Scholar]
- Quiroz-Cardoso O., Oros-Ruiz S., Solís-Gómez A., López R., Gómez R. (2019). Enhanced photocatalytic hydrogen production by CdS nanofibers modified with graphene oxide and nickel nanoparticles under visible light. Fuel 237, 227–235. 10.1016/j.fuel.2018.10.013 [DOI] [Google Scholar]
- Rahimi-Nasrabadi M., Khoshroo A., Mazloum-Ardakani M. (2017). Electrochemical determination of diazepam in real samples based on fullerene-functionalized carbon nanotubes/ionic liquid nanocomposite. Sensors Actuat B Chem 240, 125–131. 10.1016/j.snb.2016.08.144 [DOI] [Google Scholar]
- Rosman N. N., Mohamad Yunus R., Jeffery Minggu L., Arifin K., Salehmin M. N. I., Mohamed M. A., et al. (2018). Photocatalytic properties of two-dimensional graphene and layered transition-metal dichalcogenides based photocatalyst for photoelectrochemical hydrogen generation: An overview. Int. J. Hydrogen Energ. 43, 18925–18945. 10.1016/j.ijhydene.2018.08.126 [DOI] [Google Scholar]
- Sargin I., Yanalak G., Arslan G., Patir I. H. (2019). Green synthesized carbon quantum dots as TiO2 sensitizers for photocatalytic hydrogen evolution. Int. J. Hydrogen Energ. 44, 21781–21789. 10.1016/j.ijhydene.2019.06.168 [DOI] [Google Scholar]
- Smith B. W., Monthioux M., Luzzi D. E. (1998). Encapsulated C60 in carbon nanotubes. Nature 396, 323–324. 10.1038/24521 [DOI] [Google Scholar]
- Song L., Guo C., Li T., Zhang S. (2017). C60/graphene/g-C3N4 composite photocatalyst and mutually- reinforcing synergy to improve hydrogen production in splitting water under visible light radiation. Ceram. Int. 43, 7901–7907. 10.1016/j.ceramint.2017.03.115 [DOI] [Google Scholar]
- Sudhaik A., Raizada P., Shandilya P., Jeong D.-Y., Lim J.-H., Singh P. (2018). Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem. 67, 28–51. 10.1016/j.jiec.2018.07.007 [DOI] [Google Scholar]
- Sun Z., Guo J., Zhu S., Mao L., Ma J., Zhang D. (2014). A high-performance Bi2WO6-graphene photocatalyst for visible light-induced H2 and O2 generation. Nanoscale 6, 2186–2193. 10.1039/C3NR05249D [DOI] [PubMed] [Google Scholar]
- Tahir M. (2019). La-modified TiO2/carbon nanotubes assembly nanocomposite for efficient photocatalytic hydrogen evolution from glycerol-water mixture. Int. J. Hydrogen Energ. 44, 3711–3725. 10.1016/j.ijhydene.2018.12.095 [DOI] [Google Scholar]
- Tong H., Ouyang S., Bi Y., Umezawa N., Oshikiri M., Ye J. (2012). Nano-photocatalytic materials: possibilities and challenges. Adv. Mater. 24, 229–251. 10.1002/adma.201102752 [DOI] [PubMed] [Google Scholar]
- Wang G., Zhang W., Li J., Dong X., Zhang X. (2019). Carbon quantum dots decorated BiVO4 quantum tube with enhanced photocatalytic performance for efficient degradation of organic pollutants under visible and near-infrared light. J. Mater. Sci. 54, 6488–6499. 10.1007/s10853-019-03316-y [DOI] [Google Scholar]
- Wang J., Ng Y. H., Lim Y.-F., Ho G. W. (2014). Vegetable-extracted carbon dots and their nanocomposites for enhanced photocatalytic H2 production. RSC Adv. 4, 44117–44123. 10.1039/C4RA07290A [DOI] [Google Scholar]
- Wang J., Wang G., Wang X., Wu Y., Su Y., Tang H. (2019). 3D/2D direct Z-scheme heterojunctions of hierarchical TiO2 microflowers/g-C3N4 nanosheets with enhanced charge carrier separation for photocatalytic H2 evolution. Carbon 149, 618–626. 10.1016/j.carbon.2019.04.088 [DOI] [Google Scholar]
- Wang J., Wang G., Wei X., Liu G., Li J. (2018). ZnO nanoparticles implanted in TiO2 macrochannels as an effective direct Z-scheme heterojunction photocatalyst for degradation of RhB. Appl. Surf. Sci. 456, 666–675. 10.1016/j.apsusc.2018.06.182 [DOI] [Google Scholar]
- Wang Y., Chen J., Liu L., Xi X., Li Y., Geng Z., et al. (2019). Novel metal doped carbon quantum dots/CdS composites for efficient photocatalytic hydrogen evolution. Nanoscale 11, 1618–1625. 10.1039/C8NR05807E [DOI] [PubMed] [Google Scholar]
- Xia Y., Li Q., Lv K., Tang D., Li M. (2017). Superiority of graphene over carbon analogs for enhanced photocatalytic H2-production activity of ZnIn2S4. Appl. Catal. B: Environ. 206, 344–352. 10.1016/j.apcatb.2017.01.060 [DOI] [Google Scholar]
- Xiang Q., Yu J., Jaroniec M. (2011). Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J. Phys. Chem. C 115, 7355–7363. 10.1021/jp200953k [DOI] [Google Scholar]
- Xin Q., Shah H., Nawaz A., Xie W., Akram M. Z., Batool A., et al. (2018). Antibacterial carbon-based nanomaterials. Adv. Mater. 10.1002/adma.201804838. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Xu X. Y., Ray R., Gu Y. L., Ploehn H. J., Gearheart L., Raker K., et al. (2004). Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 126, 12736–12737. 10.1021/ja040082h [DOI] [PubMed] [Google Scholar]
- Yang M.-Q., Zhang N., Pagliaro M., Xu Y.-J. (2014). Artificial photosynthesis over graphene–semiconductor composites. Are we getting better? Chem. Soc. Rev. 43, 8240–8254. 10.1039/C4CS00213J [DOI] [PubMed] [Google Scholar]
- Ye A., Fan W., Zhang Q., Deng W., Wang Y. (2012). CdS–graphene and CdS–CNT nanocomposites as visible-light photocatalysts for hydrogen evolution and organic dye degradation. Catal. Sci. Tech. 2, 969–978. 10.1039/c2cy20027a [DOI] [Google Scholar]
- Ye L., Fu J., Xu Z., Yuan R., Li Z. (2014). Facile one-pot solvothermal method to synthesize sheet-on-sheet reduced graphene oxide (RGO) /ZnIn2S4 nanocomposites with superior photocatalytic performance. ACS Appl. Mater. Interfaces 6, 3483–3490. 10.1021/am5004415 [DOI] [PubMed] [Google Scholar]
- Yi H., Huang D., Qin L., Zeng G., Lai C., Cheng M., et al. (2018). Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production. Appl. Catal. B Environ. 239, 408–424. 10.1016/j.apcatb.2018.07.068 [DOI] [Google Scholar]
- Yu H., Xiao P., Tian J., Wang F., Yu J. (2016). Phenylamine-functionalized rGO/TiO2 photocatalysts: spatially separated adsorption sites and tunable photocatalytic selectivity. ACS Appl. Mater. Interfaces 8, 29470–29477. 10.1021/acsami.6b09903 [DOI] [PubMed] [Google Scholar]
- Yu H., Zhao Y., Zhou C., Shang L., Peng Y., Cao Y., et al. (2014). Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2, 3344–3351. 10.1039/c3ta14108j [DOI] [Google Scholar]
- Yu W., Xu D., Peng T. (2015). Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: a direct Z-scheme mechanism. J. Mater. Chem. A 3, 19936–19947. 10.1039/C5TA05503B [DOI] [Google Scholar]
- Zhang Q., Sun X., Ruan H., Yin K., Li H. (2017). Production of yellowemitting carbon quantum dots from fullerene carbon soot. Sci. China Mater. 60, 141–150. 10.1007/s40843-016-5160-9 [DOI] [Google Scholar]
- Zhang S., Gu P., Ma R., Luo C., Wen T., Zhao G., et al. (2019). Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: a critical review. Catal. Today 335, 65–77. 10.1016/j.cattod.2018.09.013 [DOI] [Google Scholar]
- Zhang X.-Y., Li H.-P., Cui X.-L., Lin Y. (2010). Graphene/TiO2 nanocomposites: synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 20, 2801–2806. 10.1039/b917240h [DOI] [Google Scholar]
- Zhang Y., Liu Y., Gao W., Chen P., Cui H., Fan Y., et al. (2019). MoS2 nanosheets assembled on three-way nitrogen-doped carbon tubes for photocatalytic water splitting. Front. Chem. 7:325. 10.3389/fchem.2019.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L.-P., Zhu Z.-Y., Li Y., Zhu D.-Z., Xia H.-H. (2008). Isotopic mass effects for low-energy ion channeling in single-wall carbon nanotubes. J. Phys. Chem. C 112, 15204–15206. 10.1021/jp8036999 [DOI] [Google Scholar]
- Zhou M., Dong G., Yu F., Huang Y. (2019). The deep oxidation of NO was realized by Sr multi-site doped g-C3N4 via photocatalytic method. Appl. Catal. B Environ. 256:117825 10.1016/j.apcatb.2019.117825 [DOI] [Google Scholar]