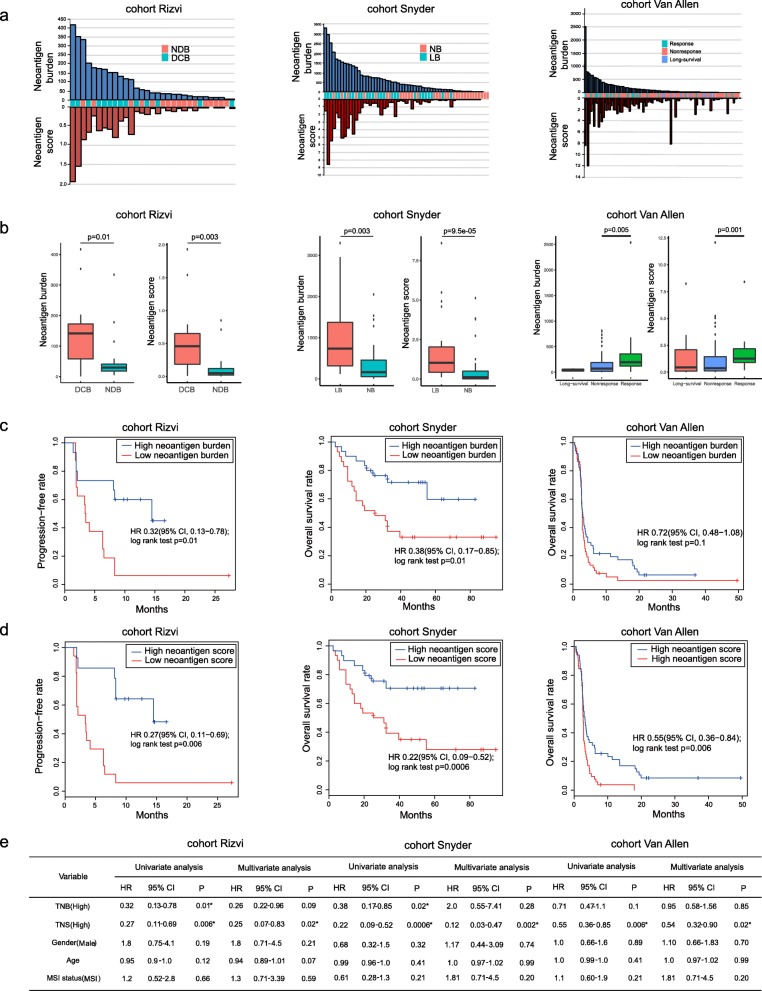
Fig. 6.
a Neoantigen burden and neoantigen immunogenicity score distribution in three immunotherapy-treated patient cohorts. b The overall neoantigen immunogenicity score showed more difference than neoantigen burden between long benefit group and no benefit group. c In cohort Rizvi and Snyder, a high neoantigen burden (> median) was associated with improved progression-free survival (PFS) or overall survival (OS) (cohort Rizvi: hazard ratio [HR] 0.32, 95% confidence interval [CI] 0.13 to 0.78, log-rank P = 0.01; cohort Snyder: HR 0.38, 95% CI 0.17 to 0.85, log-rank P = 0.01) whereas in cohort Van Allen, neoantigen burden was not associated with improved OS (HR 0.72, 95% CI 0.48 to 1.08, log-rank P = 0.1). d High overall neoantigen immunogenicity score (> median) shows more significant patient PFS or OS separations than those based purely on neoantigen burden (cohort Rizvi: HR 0.27 [95% CI, 0.11–0.69], log-rank P = 0.006; cohort Snyder: HR 0.22 [95% CI, 0.09–0.52], log-rank P = 0.0006; cohort Van Allen: HR 0.55 [95% CI, 0.36–0.84], log-rank P = 0.006). e Univariate Cox regression and multivariate Cox regression analyses showed that only overall neoantigen immunogenicity score was associated with improved PFS or OS. NDB, no durable benefit; DCB, durable clinical benefit; NB, no benefit; LB, long benefit; PFS, progression-free survival; OS, overall survival; TNB, tumor neoantigen burden; TNS, overall tumor neoantigen immunogenicity score; HR, hazard ratio; CI, confidence interval