Abstract
Background:
As the non-communicable disease (NCD) burden is rising in regions with high HIV prevalence, patients with comorbid HIV and chronic NCDs may benefit from integrated chronic disease care. There are few evaluations of the effectiveness of such strategies, especially those that directly leverage and extend the existing HIV care system to provide co-located care for NCDs.
Setting:
Academic Model of Providing Access to Healthcare (AMPATH), Kenya, provides care to over 160,000 actively enrolled patients in catchment area of 4 million people.
Methods:
Using a difference-in-differences design, we analyzed retrospective clinical records of 3603 patients with comorbid HIV and hypertension during 2009–2016 to evaluate the addition of chronic disease management (CDM) to an existing HIV care program. Outcomes were blood pressure (BP), hypertension control, and adherence to HIV care.
Results:
Compared to the HIV standard of care, the addition of CDM produced statistically significant, though clinically small improvements in hypertension control, decreasing systolic BP by 0.76mmHg (p<0.001), diastolic BP by 1.28mmHg (p<0.001), and increasing the probability of BP<140/90mmHg by 1.51 percentage points (p<0.001). However, sustained control of hypertension for >1 year improved by 7 percentage points (p<0.001), adherence to HIV care improved by 6.8 percentage points (p<0.001) and retention in HIV care with no gaps >6months increased by 10.5 percentage points (p<0.001).
Conclusion:
A CDM program that co-locates NCD and HIV care shows potential to improve blood pressure and retention in care. Further evaluation of program implementation across settings can inform how to maximize hypertension control among patients with comorbid HIV, and better understand the effect on adherence.
Keywords: Integrated care, Non-communicable disease, hypertension, adherence
Introduction
In sub-Saharan Africa (SSA), people living with HIV (PLWH) have longer survival and are aging, largely due to the success and scale up of antiretroviral treatment (ART).1 These demographic shifts for PLWH introduce new challenges to care as co-morbid non- communicable diseases (NCD) are becoming a leading cause of morbidity and mortality.2 A 2016 systematic review reported between 21–52% of PLWH in low- and middle-income countries had a comorbid cardiovascular disease risk factor.3 At the same time, there is a growing burden of NCDs in SSA in the general population, and limited availability of chronic disease care for NCDs more broadly, especially in rural and resource poor settings in SSA.1,4As HIV is increasingly recognized as a chronic disease with effective treatment, initiatives integrating treatment for both HIV and chronic NCDs have become a strategic approach for health systems to improve access to chronic disease care and manage comorbid HIV and NCDs.
Infrastructure development and lessons learned from HIV treatment and care delivery can be applied to chronic NCD care through integrated care since there are common treatment barriers and challenges.5,6 Several studies have evaluated the feasibility of integrated care programs in SSA, but research gaps remain about their implementation and efficacy, especially as the operationalization of integrated care varies considerably, and programs adding chronic NCD care to HIV care systems have further considerations that have not yet been evaluated.7–14 For example, providers of HIV care are concerned about how additional programs will impact HIV care retention and adherence.15 Patients enrolled in HIV care programs may have better knowledge and control of their chronic NCDs, so there may not be a direct health benefit for comorbid HIV and NCD patients of an integrated care that formalizes treatment of both conditions.16
This study evaluates an integrated care program in western Kenya that layered co- located chronic NCD care onto the HIV care program. Because the HIV care program was well established before the addition of chronic NCD care, we can evaluate the addition of co-located NCD care on HIV patients with comorbid hypertension and identify changes in blood pressure control, adherence and retention for either NCD or HIV care.
Methods
Study Setting and population
The Academic Model of Providing Access to Healthcare (AMPATH) is a clinical care program in western Kenya based on a collaboration between Moi Teaching and Referral Hospital, Moi University School of Medicine, and a consortium of North American Universities. AMPATH began providing HIV care to HIV infected patients in 2001, and is now the largest HIV/AIDS care program in Kenya, with over 160,00 patients actively enrolled in care in 10 counties and 55 sub-counties in western Kenya.17 The clinical protocols for managing HIV infection are consistent with WHO recommendations.18 Since its creation, AMPATH has also expanded to provide care for mental health, cardiovascular disease, maternal and child health, and more.19
Beginning in 2011, AMPATH added Chronic Disease Management (CDM) into the clinical care program, leveraging the experience in managing HIV, to address chronic NCDs. Specialized care for cardiovascular disease and risk factors is provided at a Center of Excellence located at the referral hospital, while the CDM program provides NCD care at lower-level health facilities, with nurses and clinical officers trained in diabetes and hypertension care.20 During 2011 – 2016, AMPATH added 68 CDM treatment programs to dispensaries, clinics, and hospitals throughout western Kenya, including 29 HIV clinics. These 29 clinics offered co-located HIV and NCD care, sharing infrastructure and staff, to varying degrees of provider overlap. While the CDM program provides care for chronic cardiovascular disease or diabetes regardless of HIV status even at co-located facilities, this analysis focuses on HIV patients with comorbid hypertension.
Following protocol, enrollment forms and clinical records were manually entered into the AMPATH medical records system (AMRS). Standardized forms capturing demographic, clinical, and treatment information were completed by healthcare providers at each clinical encounter. Data assistants entered these into the AMRS, with data entry validation through review of a random 10% of all forms.21,22 The dataset used in our analyses was closed in February 2017; we included data through the end of 2016 to have complete quarters of reporting only. Blood pressure (BP) was routinely measured at each clinical visit using either a manual or digital sphygmomanometer, with complete reporting for 89% of visits. Patients were considered hypertensive if they had a record of hypertensive diagnosis or were on hypertensive medications. Since the data had low levels of recorded hypertension diagnosis, but high completeness of BP records, we used measured BP to classify patients as hypertensive. According to Kenyan national guidelines, patients with persistently elevated BP of systolic BP ≥ 140, or diastolic BP ≥ 90mmHg on three separate occasions over a two-month period should be diagnosed as hypertensive.23,24 Due to variation in follow-up schedules, we extended the definition to three elevated readings within up to nine months if three measurements were not recorded within shorter periods of monitoring.
Intervention Group Definition
The intervention group consisted of patients with comorbid HIV and hypertension who received care at clinics that added co-located CDM care between 2011 and 2015, so there is at least one year of data after any implementation of CDM, irrespective of whether the individuals sought care in the co-located CDM program. Due to separate enrollment forms for CDM and AMPATH’s HIV treatment, relying on CDM records alone for the HIV patient population would have resulted in an underestimation of the number of HIV infected patients enrolled in CDM care. It would also have missed changes in care for patients who had comorbid HIV and hypertension, but did not use CDM care. By including all patients with comorbid HIV and hypertension, we also introduced fewer assumptions in the creation of the comparison group, as we did not need to predict if patients would use CDM care if it were added.
The comparison group included patients who attended HIV clinics that did not add CDM care during the study period. If patients switched between comparison and intervention clinics, they were attributed to their modal clinic for the year, and the visit at the alternative clinic was not included in the analysis, because variations in measurement may bias the results.
Inclusion and Exclusion Criteria
We used de-identified data of adult patients with HIV from the AMRS. We restricted the sample to patients who were enrolled and classified as hypertensive by 2011, and did not add patients who were enrolled later to most closely replicate the conditions of a randomized trial with defined cohorts prior to the initiation of the treatment program being studied.25 We further excluded patients who were classified as hypertensive prior to 2009, due to other changes in AMPATH programs that may introduce unmeasured confounding. Because the intervention was at the clinic level, we only analyzed patients in clinics with greater than 30 hypertensive patients enrolled during 2009–2011 for both intervention and comparison group, excluding 264 patients for a total of 3,813 patients in the analysis (see inclusion and exclusion figure in the supplemental digital content).
Ethical Review
This retrospective study used de-identified data from AMRS. Individual informed consent was not obtained. The Institutional Research and Ethics Committee of the Moi University School of Medicine and Brown University approved use of these data, and waived informed consent.
Outcomes
The outcomes of interest were changes in BP and adherence to HIV care after the addition of CDM. We are interested in a number of different BP outcomes: systolic and diastolic BP changes, controlled hypertension (BP <140/90mmHg) at each visit that both systolic and diastolic measures are available, and controlled hypertension for at least one year. Sustained control of hypertension for at least one year was the primary BP outcome measure with the most relevant potential clinical impact. Patients were considered to have controlled hypertension for at least one year if all records of BP for at least one year were <140/90mmHg. Patients without a BP record with both systolic and diastolic measurement within one year, who were not recorded as dead, were considered uncontrolled during that period due to the limited availability of hypertension treatment in the region outside of this program.4
Engagement in care is measured through adherence and retention in care outcomes. Adherence to care, is the primary adherence and retention outcome. Patients are considered adherent to care if they return to an AMPATH clinic for care within two weeks of a scheduled follow-up date, and are considered non-adherent during each quarter they remain overdue for a follow-up.26 The two-week grace period is the median length of time patients are late for a follow-up if they are still retained in care. Retention in AMPATH care reflects any clinical encounter for either HIV or NCD care. Patients who had a gap in care >6 months were considered not retained during that period. This measure of retention captures: short disengagement from care (where a patient may resume care at a later time during the follow- up period), loss to follow-up (when a patient has a gap in AMPATH care >6 months without reengagement during the follow-up period), or death.
Rigor of blood pressure measurements
BP was routinely recorded as part of AMPATH’s standard of care in the HIV clinics, yet the CDM program was associated with improved precision of BP measurement and reporting (see the supplemental digital content for details on imprecise BP measurement and reporting results in terminal digit preference (TDP) of zero, whereby significantly more measurements than statistically likely were recorded ending in zero). While it is a common problem in NCD management, TDP is considered an indicator of poor BP measurement and reporting, and can result in misclassification.27 Changes in TDP differential to intervention groups may bias evaluation results, especially because higher TDP is associated with lower mean recorded BP.28 As the proportion of BP measurements with TDP of zero varied over time, using a clinic-level covariate of the monthly proportion of BP measurements that were non-missing and did not have TDP is needed to adjust for this measurement issue.
Statistical Analysis
We used a difference-in-differences quasi-experimental design to measure the effect of implementing the CDM program on BP in HIV infected patients with comorbid hypertension.29 We analyzed the data using mixed-effects models, including clinic fixed effects, year effects to account for secular trends, and a group-period treatment indicator of CDM program initiation in treatment clinics as the difference-in-differences estimator, and controlled for changing TDP proportion. This model accounts for unmeasured confounders due to clinics, and time, across both groups, and allows for flexibility in treatment timing. We also controlled for patient-level random effects to account for repeated measurements of the same patients, and different follow-up schedules. Patient-level covariates were included to improve the precision of the standard errors. Statistical analysis was conducted with Stata 15.1, using mixed and margins.30 Separate models for hypertension control and adherence outcomes included year fixed effects to control for secular trends, a clinic indicator for clinic fixed effects, and a CDM indicator that is 1 if the clinic provided CDM care at time t, and 0 otherwise to account for variable timing of the program at different clinics.29 We used the Bonferroni correction to adjust P-values because we are examining multiple measures for each of the two main outcomes of blood pressure control and adherence. We controlled for patient-level covariates of sex, age, and WHO stage. Additionally, for hypertension control outcomes we controlled for the monthly proportion of non-TDP and non-missing BP measurements at each clinic. We stratified analysis to evaluate effect measure modification by gender, age in 10-year and 20- year age groups, and severity of hypertension at baseline, defined as Stage 2 hypertension if the first three measurements at baseline were DBP ≥ 160mmHg or DBP ≥ 100mmHg, and Stage 1 otherwise.
Sensitivity Analysis
The rollout for the intervention clinics included in the main analysis was staggered from 2011 to 2015, and while variable-treatment timing should be adequately accounted for using the method above31, if the effects of the program differ over time the staggered rollout may still introduce bias.32 Therefore, we conducted sensitivity analysis excluding intervention clinics that rolled out from 2012 onwards. A second sensitivity analysis excluded HIV patients who had WHO Stage-4 disease, due to the complications between advanced HIV disease and hypertension.33
Because adherence and retention in care includes short-term disenrollment, loss to follow-up, and death, we also conducted sensitivity analysis to isolate the effect of the added CDM program on each component repeating the analysis strategy outline above for each of these three additional outcomes. We also included sensitivity analysis of changing the grace period for adherence to care from 14 days to 3 days, 1 month, and 3 months to assess the impact on the effect on adherence to care outcome.
Results
We present patient and clinic characteristics at baseline, which is the time of patient’s hypertension classification, between 2009 and 2011 (Table 1). Among the 3,786 patients who contributed 118,894 visits to 24 clinics, the average patient age was 45 years, 43% were male, and 80% had initiated ART. On average, patients were followed for 38 visits over 5.6 years, each contributing on average 32 complete blood pressure measurements. Patient age, gender, and ART status did not differ significantly across the comparison and intervention clinics. Education level was lower at comparison clinics with 46% having either no or primary education compared to 39% of patients at CDM clinics. The difference in WHO disease stage for HIV was marginally significant between groups, as 34% of the patients in the CDM clinics had stage-1, 22% had stage-2, 35% had stage-3, and 9% had stage-4, compared with 38%, 24%, 30%, and 8% respectively. The mean systolic BP at diagnosis was 147mmHg and mean diastolic pressure was 89mmHg for the full sample and both treatment groups. The mean clinic percent of BP measures that were non-missing and non-TDP was 30% with no significant difference. Clinics in the intervention group were more likely to be larger and urban compared to the comparison clinics, which may be associated with more providers, or infrastructure support, however the difference-in-differences method accounts for these non-time varying unobservable clinic level covariates.
Table 1:
Sociodemographic and Disease Characteristics of the Patients in the CDM Clinics and Comparison Clinics at Baseline
| Full Sample | CDM Clinics | Comparison Clinics | ||
|---|---|---|---|---|
| Total Visits | 118,894 | 92,099 | 26,795 | |
| Blood Pressure Measurements | 108,583 | 83,901 | 24,682 | |
| Patients | N=3786 | N= 2904 | N=882 | |
| Patient Characteristic | N/Mean (% or sd) | N/Mean (% or sd) | N/Mean (% or sd) | p-value |
| Age | 45.0 (10.1) | 45.0 (10.0) | 45.0 (10.2) | 0.7645 |
| Male | 1594 (42%) | 1242 (43%) | 352 (40%) | 0.132 |
| On ARV | 3051 (81%) | 2345 (81%) | 706 (80%) | 0.6428 |
| Education Level | ||||
| None | 45 (1%) | 40 (1%) | 5 (1%) | 0.0021 |
| Primary | 1490 (39%) | 1091 (38%) | 399 (45%) | |
| Secondary | 532 (14%) | 425 (15%) | 107 (12%) | |
| Tertiary | 1184 (31%) | 956 (33%) | 228 (26%) | |
| Missing | 535 (15%) | 392 (13%) | 143 (16%) | |
| WHO stage | ||||
| 1 | 1336 (35%) | 1001 (34%) | 335 (38%) | 0.0501 |
| 2 | 850 (22%) | 640 (22%) | 210 (24%) | |
| 3 | 1275 (34%) | 1007 (35%) | 268 (30%) | |
| 4 | 325 (9%) | 256 (9%) | 69 (8%) | |
| Systolic Blood Pressure | 147.9 (15.0) | 147.8 (14.9) | 148.3 (15.3) | 0.3483 |
| Diastolic Blood Pressure | 89.6 (11.2) | 89.5 (11.3) | 89.9 (10.9) | 0.4638 |
| Stage 2 Hypertension (BP >160/100mmHg) | 537 (14%) | 407 (14%) | 130 (15%) | 0.5893 |
| Patient Total BP Measurements | 31.8 (13.5) | 32.3 (13.8) | 30.0 (12.3) | 0.5847 |
| Patient Total Length of Follow Up (years) | 5.58 (2.7) | 5.63 (2.7) | 5.39 (2.5) | 0.6894 |
| Clinic Characteristics | 24 Clinics | 13 Clinics | 10 Clinics | |
| Annual Visits | 16,504 (19,223) | 20,668 (20,360) | 7,481 (13,880) | 0.1709 |
| Non-Missing/Non-TDPa | 30% (40%) | 28% (40%) | 33% (40%) | 0.4076 |
| Urban clinic | 2458 (68%) | 1937 (66%) | 521 (78%) | <0.0001 |
The percentage of all blood pressures recorded in each clinic that were neither missing, nor had a terminal digit preference (TDP) of zero.
Results for the six key outcomes are presented in Table 2. The addition of CDM was associated with a decrease in systolic BP of 0.76mmHg (95% Confidence Interval: −1.14, −0.38), and a decrease in diastolic BP of 1.28mmHg (95% CI: −1.53, −1.04). The addition of CDM increased the probability of a normal BP by 1.51 percentage points (95% CI: 0.56, 2.47), however there was a much larger change in the probability they would maintain control for at least a year, which increased by 6.69 percentage points (95% CI: 6.07, 7.31). CDM improved follow-up appointment adherence by 6.76 percentage points (95% CI: 6.10,7.42), and led to an even larger significant improvement in retention in care of 10.5 percentage point increase (95% CI 10.0, 10.9). Stratified analysis did not show differences by gender or age group, and the outcomes by gender, age and for patients with stage 1 hypertension were not materially different from the main analysis (data not shown). However patients with stage 2 hypertension at baseline had larger improvements in most blood pressure control outcomes compared to the main analysis (appendix table A6). The addition of CDM was associated with a decrease in systolic BP of 3.10 mmHg (95% Confidence Interval: −4.33,−1.88), and a decrease in diastolic BP of 3.08 mmHg (95% CI: −3.83, −2.34) and increased the probability of a normal BP by 4.41 percentage points (95% CI: 1.93, 6.89). However the improvement in long term hypertension control was lower, improving by only 2.54 percentage points (95% CI: 1.49, 3.58).
Table 2:
Difference-in-Difference Outcomes for blood pressure measurement, hypertension control, and adherence to care
| Blood Pressure Measurement | Hypertension Control | Adherence to Care | ||||
|---|---|---|---|---|---|---|
| SBP (mmHg) | DBP (mmHg) | Per Visit BP <140/90mmHg |
Controlled >1 year | Adherent to Follow-up | Retained in Care | |
| Added | −0.76*** | −1.28*** | 1.51** | 6.69*** | 6.76*** | 10.5*** |
| CDMa | −1.14,−0.38] | [−1.53,−1.04] | [0.56,2.47] | [6.07,7.31] | [6.10,7.42] | [10.0,10.9] |
| Obs.b | 108,545 | 108,577 | 108,480 | 116,252 | 132,235 | 135,962 |
| Patients | 3780 | 3780 | 3780 | 3595 | 3578 | 3602 |
95% confidence intervals in [brackets]
p < 0.05
p < 0.01
p < 0.001
The Added CDM are the dif-in-dif coefficients, which control for clinic effects, secular trends, time varying covariates, and patient covariates of age, sex, WHO stage, and ARV status
Observations used in the analysis of each outcome, differences in number of observation is due to missing data for individual outcomes.
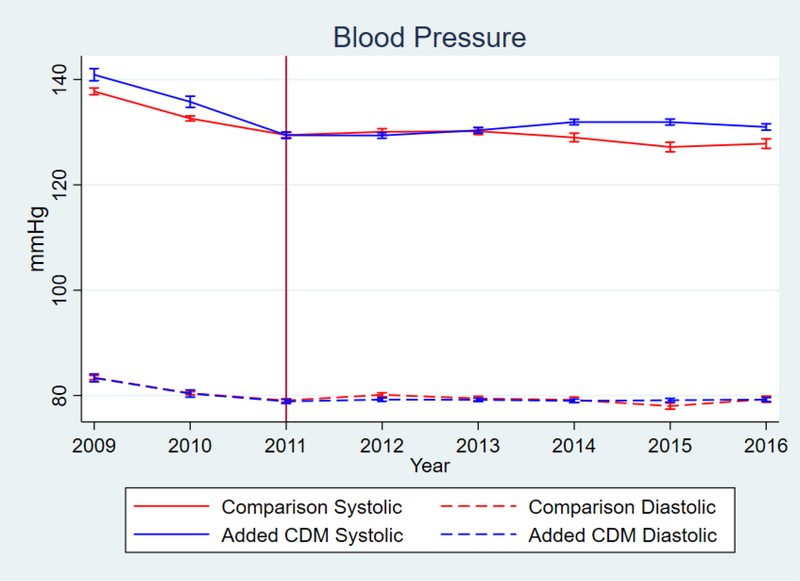
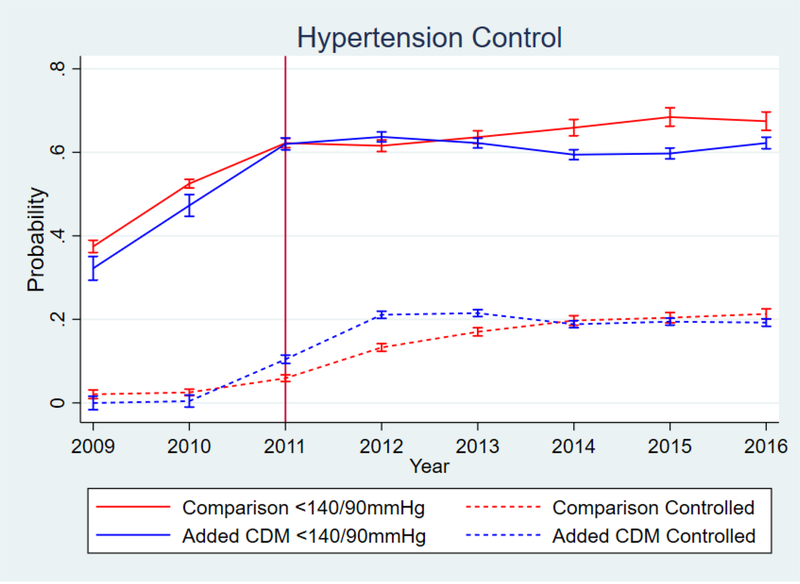
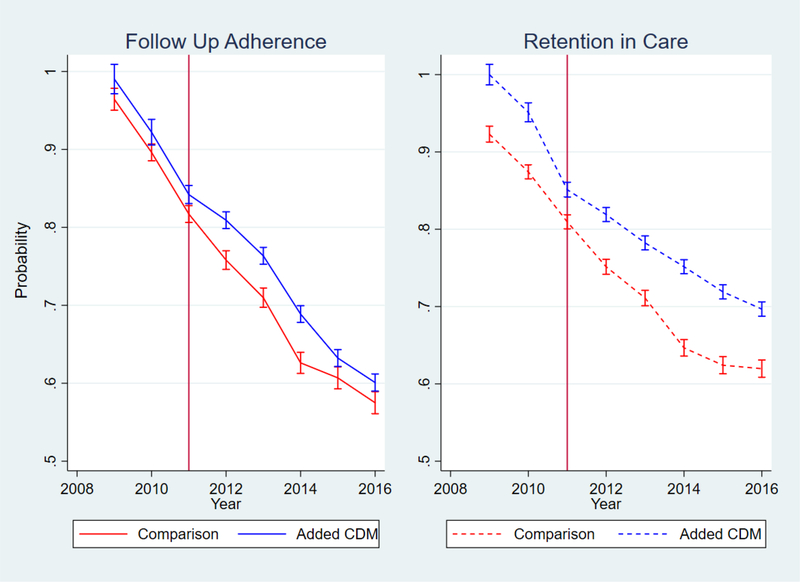
While there was improvement with the addition of the CDM program, the changes in BP and probability of BP <140/90 were small, and diminished substantially over time, as shown in the marginal effects graphs in figures 1 and 2. The 6.69 percentage-point increase in probability of hypertension control corresponds to a much larger relative difference since the baseline probability was low for both groups (figure 2); however, that benefit also attenuated over time. The graphical marginal effects of the CDM program on the adherence and retention outcomes are in figure 3. Overall CDM increased follow-up adherence for engaged patients, and the program had the largest effect on care retention. While both groups lost patients over time, clinics with added CDM had a much slower decrease compared to the comparison clinics.
Figure 1.
Blood Pressure measurements over time. Vertical Line delineates end of enrollment period
Figure 2.
Blood Pressure control over time.Vertical Line delineates end of enrollment period
Figure 3:
Probability of Retention in Care and Adherence to Follow-up
Sensitivity Analyses
Excluding patients who were enrolled in CDM clinics that rolled out later than 2011 resulted in similar results as the primary analysis (See sensitivity analysis in supplemental digital content). The clinics that initiated the CDM program earlier may have been selected for earlier rollout due to provider or administration interest in the program; however, the results were not significantly different for this restricted sample. Excluding patients with WHO disease stage-4 also resulted in similar results for our outcomes. This may be because patients with stage-4 of HIV were only 9% of our sample, exerting a limited effect on overall results. Patients with stage- 4 may make up such a small proportion of our sample because of the association of advanced HIV with wasting, resulting in lower BMI and BP, so a smaller number of patients with stage-4 would be hypertensive. Patient’s with WHO stage-4 were also similarly distributed in both treatment arms, so controlling for the clinic- and time-varying effects adequately controlled for any differences that would have been introduced. The CDM program was beneficial for all three components of retention in care. Short-term disengagement, loss to follow-up without-a- record-of-death, and mortality, all decreased with the addition of CDM. Varying the length of the grace period for adherence to care changed the magnitude of the effect, reducing it to 5.7 percentage points for a grace period of 3 days, and increasing it to 11.5 percentage points with a grace period of 3 months, but the significance and trends of the outcome was consistent.
Discussion
Evaluation of integrated and co-located chronic disease care programs are important in the development of policy in HIV/NCD care integration.15,34 Our study shows a small but significant reduction in per visit BP, and a larger significant improvement in long-term BP control (>1 year). Appointment adherence was slightly decreased for patients retained in care, but the addition of the CDM program was associated with a large increase in care retention. Furthermore, reductions in systolic, diastolic, and per visit hypertension control was much larger in magnitude for patients with Stage 2 hypertension at classification. As Stage 2 hypertension is associated with higher likelihood of adverse health outcomes, improvement in blood pressure for this group may have a larger clinical significance.
The extended length of follow-up of this study compared to prior studies of integrated chronic disease care in SSA, adds important evidence to the literature as both hypertension and HIV require life-long care.11 While per-visit measurements are important, they are insufficient if patients are regularly moving between controlled and uncontrolled hypertension, or if they have large gaps in care when they may be uncontrolled, or non-adherent. Long-term BP control suggests consistency of care, and is crucial to reduce hypertension associated morbidity and mortality. Our graphic depiction in the differences in BP between the two groups shows stabilization, and a return to similar BP in both arms during later years of the follow-up. Because loss to follow-up in this sample includes death, this may capture mortality associated with NCDs among PLHIV. In the main analysis, retention is not differentiated by mortality because deaths recorded in the AMRS understate mortality, and the comprehensiveness may vary across clinics and over time with increased focus on adherence and retention methods, introducing bias.35 However, our sensitivity analysis found a decreased mortality in reported deaths with the addition of CDM.
This long-term follow-up also provides important evidence that retention efforts for HIV patients is not diluted by adding CDM care, and is in fact benefitted by it. Both the adherence to care schedule and the retention in AMPATH care are significantly increased for the CDM arm. Throughout the follow up period AMPATH introduced other programs aimed at improving retention and adherence to care. These programs were not included in this evaluation, as their implementation the multiple clinics was not timed to coincide with the CDM program so would be absorbed as part of the external temporal trends. To our knowledge, this is the first quasi- experimental evaluation of the effectiveness of a co-located, but not fully integrated, HIV and NCD care program in SSA and the results of our study show that, even without full integration, the program shows some success in across all of the measures. Integrated chronic care in South Africa, and in Uganda and Kenya as part of the Sustainable East Africa Research for Community Health (SEARCH) Study, have shown similar effectiveness outcomes for hypertension control and retention in care.10,11,13,14 Benefits of co-located versus fully integrated care have been debated in other settings and high-income countries, but this provides a starting place for understanding co-located care as an integration strategy for chronic care in SSA.36,37 An evaluation of variation in clinical outcomes between fully integrated versus co-located HIV/NCD care may be able to extricate more differences as that evidence becomes available. Strengths and limitations
Our study has some limitations in light of working with clinical data with incomplete records. The classification of hypertension was rarely from an official diagnosis, and did not follow the traditional screening protocol of three measurements within the same appointment. However, our method reproduced the standard of hypertension evaluation for a patient in care at these clinics, including a period of monitoring BP to see if high values were anomalous, stress induced due to a recent HIV diagnosis, or could be addressed with lifestyle changes. Frequency of BP measurements was not differential to prior hypertension status or BP level. If patients were previously diagnosed as hypertensive without a record in their clinical charts, they would not be included in this study if they were well controlled. As they would have been controlled prior to the roll out of the CDM program began, it would not have an impact on our findings.
A strength of our research is the statistical method is able to control for secular trends in the data, and use a robust comparison group in the clinics that do not add the CDM program. This allowed us to account for unmeasured confounding, which is necessary since the selection of the clinics for the CDM program was not randomized. Another strength is the size and diversity of the cohort and length of follow-up. This robust data is crucial for diseases with long- term outcomes such as both HIV and hypertension. We did not restrict the analysis to patients who were enrolled in the CDM program, which provides a comprehensive analysis of the addition of CDM care. Evaluating all hypertensive patients enrolled in the clinics captures possible changes in care for hypertensive patients who do not enroll in the CDM program when it is implemented. Therefore, while the program level benefits are moderate, they may be improved by focusing on enrolling more patients directly in the hypertension care component of the co-located programs. As all patients included in this analysis were already enrolled in the HIV care program, they may have better hypertension awareness and control than the general population, as has been found in other settings as well, so the magnitude of the effect for patients not already enrolled in care may be higher than those that we found.16 The differential changes in both systolic and diastolic pressure, and hypertension control for patients with higher blood pressure, also suggests that the clinical benefit for this program is most substantial for higher risk patients. Finally, our data represent the real-world experience of a large HIV care program in SSA, which enhances the generalizability of our findings to similar programs in the region.
Conclusion
Integrated HIV and NCD care is an important health systems strategy for strengthening chronic disease care. Our study contributes to the understanding of the impact of integrated care to the healthcare of HIV-positive patients with comorbid hypertension. While adding co- located NCD care to an existing care program has potential risks, this extant evidence suggests that this method provides moderate benefits in hypertension control and long-term adherence to care for these patients.
Supplementary Material
Source of Funding:
This work was funded through NIH/Fogarty International Center; PEPFAR; CRDF Global (OISE- 17-62967-1). We are grateful to the Population Studies and Training Center at Brown University, which receives funding from the NIH (P2C-HD-041020), for general support. This work was also facilitated by the Providence/Boston Center for AIDS Research (P30-AI-042853).
Footnotes
The authors have no conflicts to declare
Supplemental Digital Content:
References
- 1.Agyepong IA, Sewankambo N, Binagwaho A, et al. The path to longer and healthier lives for all Africans by 2030: the Lancet Commission on the future of health in sub-Saharan Africa. Lancet. 2017;390(10114):2803–2859. doi: 10.1016/S0140-6736(17)31509-X [DOI] [PubMed] [Google Scholar]
- 2.Duffy M, Ojikutu B, Andrian S, Sohng E, Minior T, Hirschhorn LR. Non-communicable diseases and HIV care and treatment: models of integrated service delivery. Trop Med Int Heal. 2017;22(8):926–937. doi: 10.1111/tmi.12901 [DOI] [PubMed] [Google Scholar]
- 3.Patel P, Rose CE, Collins PY, et al. Noncommunicable diseases among HIV-infected persons in low-income and middle-income countries. AIDS. 2018;32:S5–S20. doi: 10.1097/QAD.0000000000001888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moucheraud C Service readiness for noncommunicable diseases was low in five countries in 2013–15. Health Aff. 2018;37(8):1321–1330. doi: 10.1377/hlthaff.2018.0151 [DOI] [PubMed] [Google Scholar]
- 5.Bloomfield GS, Alenezi F, Barasa FA, Lumsden R, Mayosi BM, Velazquez EJ. Human Immunodeficiency Virus and Heart Failure in Low- and Middle-Income Countries. JACC Hear Fail. 2015;3(8):579–590. doi: 10.1016/j.jchf.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rachlis B, Naanyu V, Wachira J, et al. Identifying common barriers and facilitators to linkage and retention in chronic disease care in western Kenya. BMC Public Health. 2016;16:741. doi: 10.1186/s12889-016-3462-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wroe EB, Kalanga N, Mailosi B, et al. Leveraging HIV platforms to work toward comprehensive primary care in rural Malawi: the Integrated Chronic Care Clinic. Healthcare. 2015;3(4):270–276. doi: 10.1016/j.hjdsi.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 8.Edwards JK, Bygrave H, Van den Bergh R, et al. HIV with non-communicable diseases in primary care in Kibera, Nairobi, Kenya: characteristics and outcomes 2010–2013. Trans R Soc Trop Med Hyg. 2015;109(7):440–446. doi: 10.1093/trstmh/trv038 [DOI] [PubMed] [Google Scholar]
- 9.Topp SM, Chipukuma JM, Giganti M, et al. Strengthening Health Systems at Facility-Level: Feasibility of Integrating Antiretroviral Therapy into Primary Health Care Services in Lusaka, Zambia. Myer L, ed. PLoS One. 2010;5(7):e11522. doi: 10.1371/journal.pone.0011522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawat A, Uebel K, Moore D, Yassi A. Integrated HIV-Care Into Primary Health Care Clinics and the Influence on Diabetes and Hypertension Care: An Interrupted Time Series Analysis in Free State, South Africa Over 4 Years.; 2018. http://links.lww.com/QAI/B115. Accessed October 30, 2018. [DOI] [PubMed]
- 11.Ameh S, Klipstein-Grobusch K, Musenge E, Kahn K, Tollman S, Gómez-Olivé FX. Effectiveness of an Integrated Approach to HIV and Hypertension Care in Rural South Africa: Controlled Interrupted Time-Series Analysis. J Acquir Immune Defic Syndr. 2017;75(4):472–479. doi: 10.1097/QAI.0000000000001437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Njuguna B, Vorkoper S, Patel P, et al. Models of integration of HIV and noncommunicable disease care in sub-Saharan Africa. AIDS. 2018;32:S33–S42. doi: 10.1097/QAD.0000000000001887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwarisiima D, Atukunda M, Owaraganise A, et al. Hypertension control in integrated HIV and chronic disease clinics in Uganda in the SEARCH study. BMC Public Health. 2019;19(1):511. doi: 10.1186/s12889-019-6838-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwarisiima D, Kamya MR, Owaraganise A, et al. High rates of viral suppression in adults and children with high CD4+ counts using a streamlined ART delivery model in the SEARCH trial in rural Uganda and Kenya. J Int AIDS Soc. 2017;20(Suppl 4):21673. doi: 10.7448/IAS.20.5.21673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matanje Mwagomba BL, Ameh S, Bongomin P, et al. Opportunities and challenges for evidence-informed HIV-noncommunicable disease integrated care policies and programs. AIDS. 2018;32:S21–S32. doi: 10.1097/QAD.0000000000001885 [DOI] [PubMed] [Google Scholar]
- 16.Manne-Goehler J, Montana L, Gómez-Olivé FX, et al. The ART Advantage: Health Care Utilization for Diabetes and Hypertension in Rural South Africa. J Acquir Immune Defic Syndr. 2017;75(5):561–567. doi: 10.1097/QAI.0000000000001445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rachlis B, Naanyu V, Wachira J, et al. Identifying common barriers and facilitators to linkage and retention in chronic disease care in western Kenya. BMC Public Health. 2016;16:741. doi: 10.1186/s12889-016-3462-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wools-Kaloustian K, Kimaiyo S, Diero L, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS. 2006;20(1):41–48. doi: 10.1097/01.aids.0000196177.65551.ea [DOI] [PubMed] [Google Scholar]
- 19.Einterz RM, Kimaiyo S, Mengech HNK, et al. Responding to the HIV Pandemic: The Power of an Academic Medical Partnership. Acad Med. 2007;82(8):812–818. doi: 10.1097/ACM.0b013e3180cc29f1 [DOI] [PubMed] [Google Scholar]
- 20.Binanay CA, Akwanalo CO, Aruasa W, et al. Building Sustainable Capacity for Cardiovascular Care at a Public Hospital in Western Kenya. J Am Coll Cardiol. 2015;66(22):2550–2560. doi: 10.1016/j.jacc.2015.09.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siika AM, Rotich JK, Simiyu CJ, et al. An electronic medical record system for ambulatory care of HIV-infected patients in Kenya. Int J Med Inform. 2005;74(5):345–355. doi: 10.1016/J.IJMEDINF.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 22.Tierney WM, Rotich JK, Hannan TJ, et al. The AMPATH medical record system: creating, implementing, and sustaining an electronic medical record system to support HIV/AIDS care in western Kenya. Stud Health Technol Inform. 2007;129(Pt 1):372–376. http://www.ncbi.nlm.nih.gov/pubmed/17911742. Accessed July 13, 2015. [PubMed] [Google Scholar]
- 23.Whitworth JA, World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983–1992. doi: 10.1097/01.hjh.0000084751.37215.d2 [DOI] [PubMed] [Google Scholar]
- 24.Division of Non-Communicable Diseases-Ministry of Health. Kenya National Guidelines for Cardiovascular Diseases Management.; 2018. www.health.go.ke. Accessed February 26, 2019.
- 25.Toh S, Manson JE. An analytic framework for aligning observational and randomized trial data: Application to postmenopausal hormone therapy and coronary heart disease. Stat Biosci. 2013;5(2). doi: 10.1007/s12561-012-9073-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Hogan JW, Genberg BL, et al. A state transition framework for patient-level modeling of engagement and retention in HIV care using longitudinal cohort data. Stat Med. 2018;37(2):302–319. doi: 10.1002/sim.7502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nietert PJ, Wessell A, Feifer C, Ornstein SM. Effect of Terminal Digit Preference on Blood Pressure Measurement and Treatment in Primary Care. Am J Hypertens. 2006;19(2):147–152. doi: 10.1016/j.amjhyper.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 28.Harrison WN, Lancashire RJ, Marshall TP. Variation in recorded blood pressure terminal digit bias in general practice. J Hum Hypertens. 2008;22(3):163–167. doi: 10.1038/sj.jhh.1002312 [DOI] [PubMed] [Google Scholar]
- 29.Wing C, Simon K, Bello-Gomez RA. Designing Difference in Difference Studies: Best Practices for Public Health Policy Research Keywords. Annu Rev Public Heal. 2018;39:453–469. doi: 10.1146/annurev-publhealth [DOI] [PubMed] [Google Scholar]
- 30.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. StataCorp LP; 2012. https://econpapers.repec.org/bookchap/tsjspbook/mimus2.htm. Accessed March 29, 2019. [Google Scholar]
- 31.Goodman-Bacon A Difference-in-Differences with Variation in Treatment Timing.; 2018. doi: 10.3386/w25018 [DOI]
- 32.Callaway B, C Sant PH. Difference-in-Differences with Multiple Time Periods and an Application on the Minimum Wage and Employment *.; 2018. https://arxiv.org/pdf/1803.09015.pdf. Accessed November 27, 2018.
- 33.Mohamed SF, Mutua MK, Wamai R, et al. Prevalence, awareness, treatment and control of hypertension and their determinants: results from a national survey in Kenya. BMC Public Health. 2018;18(Suppl 3):1219. doi: 10.1186/s12889-018-6052-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vorkoper S, Kupfer LE, Anand N, et al. Building on the HIV chronic care platform to address noncommunicable diseases in sub-Saharan Africa. AIDS. 2018;32:S107–S113. doi: 10.1097/QAD.0000000000001898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinkhof MWG, Spycher BD, Yiannoutsos C, et al. Adjusting mortality for loss to follow- up: analysis of five ART programmes in sub-Saharan Africa. PLoS One. 2010;5(11):e14149. doi: 10.1371/journal.pone.0014149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonciani M, Schäfer W, Barsanti S, Heinemann S, Groenewegen PP. The benefits of co- location in primary care practices: the perspectives of general practitioners and patients in 34 countries. BMC Health Serv Res. 2018;18(1):132. doi: 10.1186/s12913-018-2913-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawn S, Lloyd A, King A, Sweet L, Gum L. Integration of primary health services: being put together does not mean they will work together. BMC Res Notes. 2014;7(1):66. doi: 10.1186/1756-0500-7-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.