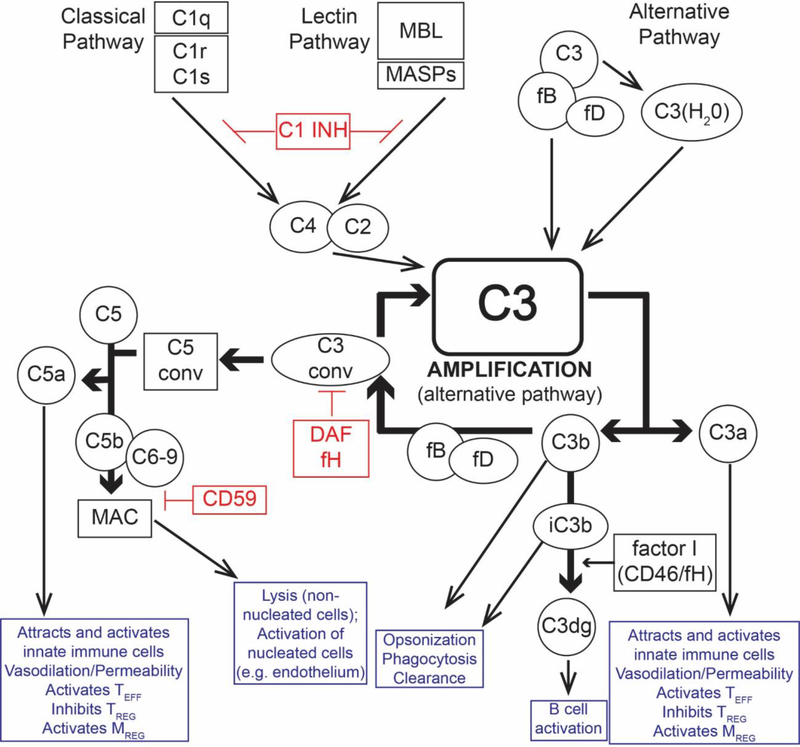
Figure 1:
Overview of the complement cascade and its diverse effects of on alloimmunity and inflammation. Complement activation can be initiated by the C1q-initiated classical pathway, the MASP-dependent mannose binding lectin (MBL) pathway, and spontaneous activation of the alternative pathway. All three pathways converge at the central amplification loop, which forms C3 convertases that cleave C3 to C3a and C3b, the latter initiating formation of the C5 convertase. Subsequent C5 cleavage yields C5a and C5b, ultimately forming the membrane attack complex (MAC, C5b-9), which activates nucleated cells (e.g. endothelium and parenchyma) and induces cytolysis in non-nucleated cells (e.g. red blood cells, bacteria). The anaphylatoxins C3a and C5a bind their respective receptors on immune and parenchymal cells to induce local inflammation, activate effector T cells, and TREG. C5a/C5aR1 paradoxically activates regulatory myeloid cells required for transplant tolerance in mice. The opsonin C3b and iC3b promote phagocytosis. iC3b also ligates complement receptor 2 (CD21) promoting B cell activation. Complement activation/amplification is restrained on self-cells by several membrane-bound and soluble regulatory proteins. Surface-expressed regulators include decay accelerating factor (DAF or CD55, accelerates the decay of cell-surface assembled C3 convertases), membrane cofactor protein (MCP or CD46, a co-factor for factor I that cleaves C3b to its inactive form, iC3b), and CD59 (protectin, inhibits formation of the MAC). Factor H is a soluble complement regulator that exhibits both decay accelerating and co-factor activity. C1 inhibitor (C1INH) inhibits C1qrs and MBL-MASP complexes, limiting classical pathway and MBL pathway activation, respectively. C1INH: C1 inhibitor; C3 conv: C3 convertase; C5 conv: C5 convertase; DAF: decay accelerating factor (CD55), fB: factor B; fD: factor D; fH: factor H; fI: factor I; MAC: membrane attack complex C5b-9; MBL: mannose-binding lectin; MASP: mannose-associated serine protease; TREG: Regulatory T cell