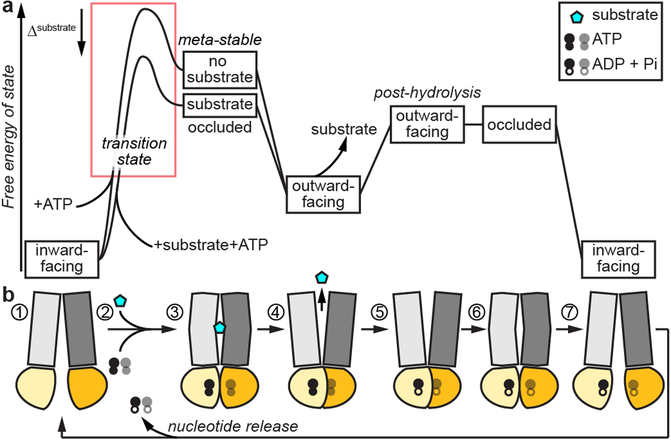
Fig. 3. Consensus thermodynamic model for transport by ABC exporters.
Vertically aligned (a) energy diagram and (b) conformational state schematics for transport cycle of type I ABC exporters. The resting state (1) is inward open. Substrate and ATP binding promotes transition through substrate-bound occluded states (2, 3), to a locally stable outward-open state (4), with an ATP-dependent NBD dimer. Substrate-bound transporters have a lower transition energy (red box), accounting for the observed substrate stimulation. After substrate release (5), ATP hydrolysis destabilizes the NBD dimer and outward-open state, leading the transporter to transition through an occluded state (6) and ending at the inward-open state (7) concomitant with release nucleotide, resetting for another round of transport.