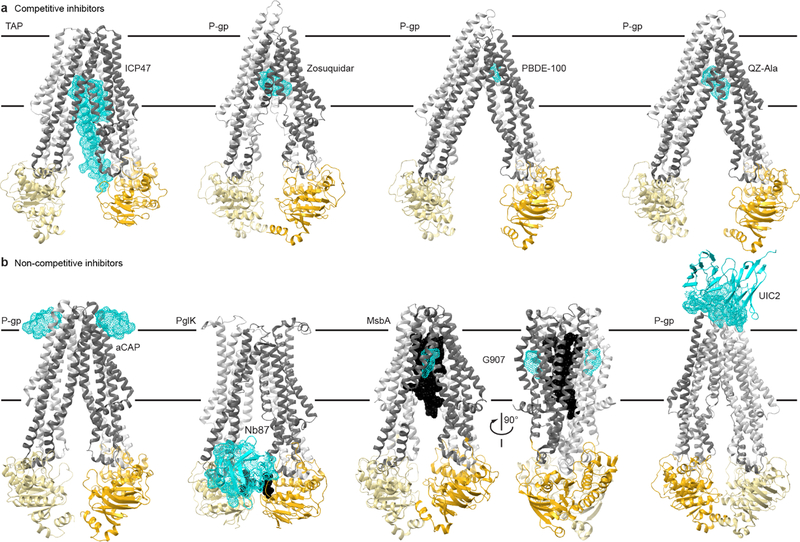
Fig. 4. Structural insights into ABC transporter pharmacology.
(a) Structures of type I exporters bound to competitive inhibitors. From left: the core domains of human TAP bound to ICP47 (PDB 5U1D); chimeric mouse-human P-gp bound to two Zosuquidar molecules, a small-molecule inhibitor (PDB 6QEE); mouse P-gp bound to the marine pollutant PBDE-100 (PDB 4XWK); mouse P-gp bound to two QZ-Ala cyclopeptides (PDB 4Q9I), which may be a competing substrate. (b) Structure of type I exporters bound to non-competitive inhibitors. From left: C. merolae P-gp homodimer bound to two aCAP cyclopeptides (PDB 3WMG); C. jejuni PglK homodimer bound to a single Nb87 nanobody (PDB 5NBD); E. coli MsbA homodimer bound to LPS substrate (black) and two G907 small-molecule inhibitors (PDB 6BPL); chimeric mouse-human P-gp bound to the UIC2 antibody (only the Fv fragment is illustrated and the transporter is rotated ~180° relative to other panels to better view the interface; PDB 6FN4). Color-coding as in Fig. 1, with the inhibitors illustrated as cyan dotted surfaces.