Abstract
Objective
To evaluate clinicopathologic factors and adjuvant treatment effects on recurrence free (RFS) and overall survival (OS) in early stage uterine clear cell carcinoma (UCCC).
Methods
Our retrospective review included central pathology confirmed stage I or II UCCC treated and/or followed between 2000 and 2016. Cases with pure or mixed histology with >50% UCCC were included. Data were analyzed using Kaplan-Meier method and Cox proportional hazards regressions.
Results
112 women were identified. Median age was 65.5 years (range 34-94). Most patients had mixed UCCC (61%), while 39% had pure UCCC. The majority of patients had stage IA UCCC (66%) versus stage IB (15%) or stage II (18%) disease. Adjuvant treatment included chemotherapy + radiation (26%), brachytherapy (27%), whole pelvic radiation (15%), chemotherapy alone (8%), and observation (24%). Thirty-eight (34%) women had recurrent disease. Median RFS was 4.32 years (95% CI 2.77 – 5.78). On multivariate analysis, age ≥ 70 (HR 2.48, 95% 1.28-4.81) and positive LVSI (HR 2.19, 95% CI 1.15-4.18) were associated with shorter RFS. Median OS was 9.8 years (95% CI 7.46-15.93). On multivariate analyses, age ≥ 70 (HR 3.57, 95% CI 1.64-7.74) and positive LVSI (HR 2.46, 95% CI 1.12-5.37) were associated with shorter OS. In this retrospective descriptive uncontrolled patient series, adjuvant treatment type did not impact RFS or OS.
Conclusions
OS approaches 10 years for early stage UCCC patients. Women ≥ 70 years have worse PFS and OS regardless of treatment modality, encouraging consideration of quality of life implications when electing for adjuvant therapy.
INTRODUCTION
Uterine clear cell carcinoma (UCCC) accounts for 1 to 6% of all endometrial cancers and is classified as a high-risk subtype [1–3]. In up to 40% of cases with clinical stage I disease, extra-uterine metastasis identified at the time of surgery [2, 4]. Similarly, in patients with stage IA disease confined to the inner one-half of the myometrium, 45% had extra-uterine disease [4]. Although considered aggressive tumors, early stage UCCCs have 5-year overall survival rates ranging from 71-79% [5–7].
Due to the rarity of these tumors, prospective trials for UCCC are lacking. Comprehensive surgical staging including a hysterectomy, bilateral salpingo-oophorectomy, lymph node assessment and omentectomy is recommended as first line treatment [5]. According to the National Comprehensive Cancer Network (NCCN) guidelines, the adjuvant treatment for stage IA UCCC can include observation, if no residual disease is identified in the hysterectomy specimen, chemotherapy with or without vaginal brachytherapy or external beam radiation therapy with or without vaginal brachytherapy. Stage IB or II disease can be treated with chemotherapy with or without external beam radiation therapy and with or without vaginal brachytherapy [8].
Given the wide-ranging acceptable adjuvant therapies for early stage UCCC, research has sought to elucidate the most beneficial treatment approach [9]. The conclusions of these studies are limited as they are retrospective, include patients with all stages of disease, or combine data from patients with clear cell or serous histology. Our objective was to examine a cohort of patients from a single institution with pathologically confirmed UCCC to determine if certain clinicopathologic factors or adjuvant treatment type were associated with improved recurrence-free (RFS) or overall survival (OS).
METHODS
Following Institutional Review Board approval (PA16-0440), a retrospective review was conducted at the University of Texas MD Anderson Cancer Center. All cases of stage I or II pure clear cell carcinoma or mixed clear cell carcinoma (with >50% of the specimen containing clear cell carcinoma) pathologically reviewed from January 2000 to April 2016 were included. Patients were excluded if their specimen contained a foci, small amount or < 50% of clear cell carcinoma. Most patient received their treatment at MD Anderson, while others were treated at outside facilities and chose to get subsequent care at MD Anderson. All patients had at least six months of follow-up post diagnosis; follow-up included clinic visits of tumor registry inquiries. Pathologic diagnosis was made or confirmed by MD Anderson gynecologic pathologists at the time of initial patient consultation. Demographic, surgical, pathologic, treatment, and follow-up information were abstracted from the medical record. Surgery consisted of a hysterectomy and removal of the adnexa if present. The extent of surgical staging, including pelvic and para-aortic lymphadenectomy, cytology, and omentectomy, was at the discretion of the attending surgeon. Stage was assigned using the International Federation of Gynecology and Obstetrics (FIGO) 2009 staging system. The type of adjuvant treatment was determined using NCCN guidelines and physician discretion; options included: observation, chemotherapy alone, chemotherapy plus radiation (whole pelvic radiation or brachytherapy), pelvic radiation with or without sensitizing chemotherapy, or brachytherapy alone.
Demographic, clinical, and pathological characteristics were summarized using standard summary statistics. OS and RFS were estimated with the product-limit estimator of Kaplan and Meier for the various levels of risk factors. Cox proportional hazards regression was used to assess the statistical significance of these potential prognostic factors for OS and RFS. From the Cox model we estimated the hazard ratio for each potential prognostic factor with a 95% confidence interval. We examined each potential prognostic factor in a univariate fashion. OS was calculated as the time from diagnosis to date of death or date of last contact. RFS was calculated as the time from diagnosis to the date or recurrence, death, or last clinic assessment of recurrence. Subset analysis examining overall survival was performed including only patients with lymph node assessment. Stata/MP v15.0 (College Station, TX) was used for all analyses. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at MD Anderson[10].
RESULTS
Patient characteristics
A total of 112 subjects were included. Table 1 includes the demographic and clinical characteristics of the study population. The median age at diagnosis was 65 years (range 34-94 years). The median BMI was 30.2 kg/m2 (range 16.9 – 64.4 kg/m2). Most patients were Caucasian (n=78, 70%) and had stage IA UCCC (n=73, 66%) versus stage IB (n=17, 15%) or stage II (n=20, 18%) disease. Mixed UCCC (n=68, 61%) was the most common histology, compared to pure UCCC (n=44, 39%). In mixed UCCC, other histological components included: endometrioid (n=30, 44%), serous (n=23, 39%), endometrioid and serous (n=14, 21%), and smooth muscle tumor of uncertain malignant potential (STUMP) (n=1, 1%). At the time of hysterectomy, lymph node assessment was completed in 81% cases (n=91). Eight-nine patients (79%) underwent a pelvic lymphadenectomy; median lymph node removal of 10 lymph nodes (range: 1-41). Seventy patients (62%) had a para-aortic lymphadenectomy, with the median number of lymph nodes removed equaling 5.5 (range: 1-22). Sixty-eight patients (60%) had both pelvic and para-aortic lymphadenectomies.
Table 1:
Demographic, clinical, and treatment characteristics
| Characteristic | n=112 |
|---|---|
| Mean Age, years (Range) | 65.1 (34 - 94) |
| Mean Weight, kg (Range) | 76.7 (46.9 – 147) |
| Mean BMI, kg/m2 (Range) | 30.2 (16.9 – 64.4) |
| Race, n (%) | |
| Caucasian | 78 (70) |
| African American | 13 (12) |
| Asian | 3 (3) |
| Other | 5 (4) |
| Unknown | 11 (10) |
| FIGO stage, n (%) | |
| Stage IA without myometrial invasion | 32 (29) |
| Stage IA with myometrial invasion | 41 (37) |
| Stage IB | 17 (15) |
| Stage II | 20 (18) |
| Histology, n (%) | |
| Mixed histology | 68 (61) |
| Pure clear cell | 44 (39) |
| Lymphadenectomy, n (%) | |
| Yes | 91 (81) |
| No | 21 (19) |
| Adjuvant treatment, n (%) | |
| Brachytherapy | 30 (27) |
| Chemotherapy plus radiation* | 29 (26) |
| Observation | 27 (24) |
| Pelvic radiation** | 17 (15) |
| Chemotherapy | 9 (8) |
| Recurrence, n (%) | |
| No | 74 (66) |
| Yes | 38 (34) |
| Recurrence location, n (%) | |
| Distant | 20 (54) |
| Pelvic | 9 (24) |
| Nodal | 8 (22) |
| Unknown | 1 (3) |
Note:
Chemotherapy plus radiation includes chemotherapy plus brachytherapy or pelvic radiation
Pelvic radiation with or without concurrent sensitizing chemotherapy
Treatment
Following surgery, 30 patients (27%) received brachytherapy alone, 29 (26%) received a combination of chemotherapy (n=26 platinum/taxane; n=3 taxane) and radiation (n=22 brachytherapy; n=7 whole pelvic radiation), 17 (15%) underwent pelvic radiation with (n=3 cisplatin) or without (n=14) sensitizing chemotherapy, 9 (8%) received chemotherapy alone (n=8 platinum/taxane combination; n=1 unknown), and 27 (24%) had no adjuvant treatment (Table 1). A higher proportion of pure clear cell patients compared to mixed histology patients received no adjuvant therapy (63% versus 37%, p=0.04, Table 2).
Table 2:
Location of recurrence and mixed or pure clear cell carcinoma CCC) by treatment group
| Characteristic | Observation | Chemo* Alone | Chemo + Radiation | Radiation | Brachytherapy | p-value |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Histology | 0.042 | |||||
| Mixed | 10 (37) | 5 (56) | 22 (76) | 12 (71) | 19 (63) | |
| Pure CCC | 17 (63) | 4 (44) | 7 (24) | 5 (29) | 11 (37) | |
| Recurrence location | 0.911 | |||||
| Pelvic | 1 (14) | 1 (20) | 1 (11) | 3 (33) | 3 (43) | |
| Nodal | 2 (29) | 1 (20) | 3 (33) | 1 (11) | 1 (14) | |
| Distant | 4 (57) | 3 (60) | 5 (56) | 5 (56) | 3 (43) | |
Chemo= Chemotherapy; Mixed histology group was more likely to received adjuvant treatment compared to the pure UCCC group. Pelvic recurrence locations were not related to type of treatment received.
Recurrence and survival
The median follow-up time for all subjects was 3.81 years (range 0.28 – 22.14 years). Thirty-eight patients were followed for longer than 5 years after their diagnosis. Thirty-eight women (34%) developed recurrent disease throughout the study period. Most recurrences (54%) were located in distant locations, followed by pelvic (24%), nodal (22%), and one site of recurrence was unknown. The location of recurrence was not associated with type of treatment received (p=0.91, Table 2).
Median RFS was 4.32 years (95% CI 2.77 – 5.78). There was no difference between the median RFS for the no adjuvant treatment group (2.77 years) and any adjuvant therapy group (4.71 years) (p=0.83, 95% CI 0.52 – 1.71). Table 3 includes the univariate and multivariable analysis for RFS results by risk factors. Age ≥ 70 years was associated with a higher risk of recurrence in the univariate and multivariate analyses (HR 2.32, 95% CI 1.36 – 3.96, p=0.002; HR 2.48, 95% CI 1.28 – 4.81, p=0.007; respectively). In the multivariate analysis, lymphovascular space invasion (LVSI) was associated with decreased survival (HR 2.19, 95% CI 1.15 – 4.18, p=0.017).
Table 3:
Univariate and multivariable recurrence – free survival
| Characteristic | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age at diagnosis (years) | ||||||
| < 70 | reference | reference | ||||
| ≥ 70 | 2.32 | (1.36, 3.96) | 0.002 | 2.48 | (1.28, 4.81) | 0.007 |
| Histology | ||||||
| Mixed | reference | reference | ||||
| Pure clear cell carcinoma | 1.31 | (0.77, 2.24) | 0.324 | 1.06 | (0.55, 2.02) | 0.866 |
| FIGO stage | ||||||
| IA | reference | reference | ||||
| IB | 2.08 | (1.01, 4.28) | 0.098 | 1.71 | (0.79, 3.70) | 0.345 |
| II | 1.54 | (0.81, 2.95) | 1.37 | (0.63, 2.99) | ||
| Lymphadenectomy | ||||||
| Yes | reference | reference | ||||
| No | 0.98 | (0.5, 1.92) | 0.961 | 0.69 | (0.32, 1.47) | 0.332 |
| LVSI | ||||||
| No | reference | reference | ||||
| Yes | 1.60 | (0.93, 2.75) | 0.088 | 2.19 | (1.15, 4.18) | 0.017 |
| Treatment | ||||||
| Observation | reference | reference | ||||
| Chemotherapy | 2.00 | (0.71, 5.65) | 0.243 | 2.36 | (0.81, 6.92) | 0.130 |
| Chemotherapy + radiation | 0.59 | (0.25, 1.35) | 0.47 | (0.19, 1.19) | ||
| Radiation | 1.21 | (0.57, 2.61) | 0.85 | (0.36, 2.05) | ||
| Brachytherapy | 0.93 | (0.45, 1.91) | 0.83 | (0.37, 1.83) | ||
The median RFS was 4.32 years (95% CI 2.77 – 5.78). Age ≥ 70 years was associated with a higher risk of recurrence in the univariate and multivariable analyses. The presence of LVSI had was associated with shorter RFS in the multivariable analysis. No other clinicopathologic features were associated with RFS.
Median overall survival of the cohort was 9.80 years (95% CI 7.46 – 15.93). Table 4 includes overall survival results by risk factors. Patients with age ≥70 years (HR 3.37, 95% CI 1.78 – 6.40, p < 0.001) had a higher risk of death. Pure clear cell carcinoma (HR 2.41, 95% CI 1.28 – 4.55, p = 0.007) was also associated with higher risk of death (Figure 1). Median OS did not vary based on adjuvant treatment type (p=0.31; Figure 2). The median OS for those not receiving treatment was shorter than those receiving adjuvant treatment (7.52 years versus 11.29 years), but this difference was not statistically significant (p=0.098, 95% CI 0.29 – 1.1). The overall survival of those patients who had lymph node assessment (n=91) did not differ based on receipt of treatment (8.3 months without treatment and 9.8 months with any adjuvant treatment, p=0.63).
Table 4.
Univariate and multivariable overall survival
| Characteristic | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age at diagnosis (years) | ||||||
| < 70 | reference | reference | ||||
| ≥ 70 | 3.37 | (1.78, 6.40) | <0.001 | 3.57 | (1.64, 7.74) | 0.001 |
| Histology | ||||||
| Mixed | reference | reference | ||||
| Pure clear cell carcinoma | 2.41 | (1.28, 4.55) | 0.007 | 1.58 | (0.75, 3.36) | 0.232 |
| FIGO stage | ||||||
| IA | reference | reference | ||||
| IB | 0.82 | (0.31, 2.15) | 0.814 | 0.60 | (0.21, 1.75) | 0.296 |
| II | 0.17 | (0.54, 2.50) | 1.52 | (0.62, 3.73) | ||
| Lymphadenectomy | ||||||
| Yes | reference | reference | ||||
| No | 0.77 | (0.32, 1.85) | 0.557 | 0.49 | (0.17, 1.40) | 0.183 |
| LVSI | ||||||
| No | reference | reference | ||||
| Yes | 1.31 | (0.69, 2.51) | 0.411 | 2.46 | (1.12, 5.37) | 0.024 |
| Treatment | ||||||
| Observation | reference | reference | ||||
| Chemotherapy | 0.63 | (0.14, 2.81) | 0.306 | 0.81 | (0.17, 3.88) | 0.442 |
| Chemotherapy+radiation | 0.29 | (0.09, 0.88) | 0.34 | (0.10, 1.16) | ||
| Radiation | 0.70 | (0.29, 1.67) | 0.51 | (0.19, 1.39) | ||
| Brachytherapy | 0.70 | (0.32, 1.54) | 0.63 | (0.25, 1.56) | ||
The median overall survival was 9.80 years (95% CI 7.46 – 15.93). Age ≥ 70 years was associated with a shorter OS in the univariate and multivariable analyses. The pure UCCC had a lower rated of OS than the mixed histology group in the univariate analysis. The presence of LVSI had was associated with shorter OS in the multivariable analysis. No other clinicopathologic features were associated with OS.
Figure 1: Kaplan Meier curve of overall survival by histology.
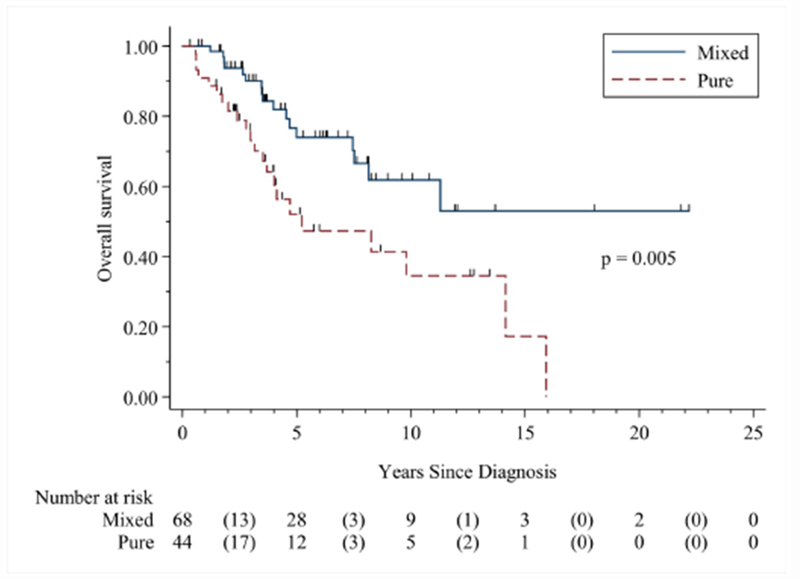
The median follow-up time was 3.81 years (range 0.28 to 22.14 months). The median overall survival for the pure UCCC histology group was 5.32 years, while the medial overall survival for the pure UCCC group was not reached. The pure UCCC group had worse overall survival than the mixed histology group (HR 2.41 (95% CI, 1.28, 4.55)).
Figure 2: Kaplan Meier curve of overall survival by treatment type.
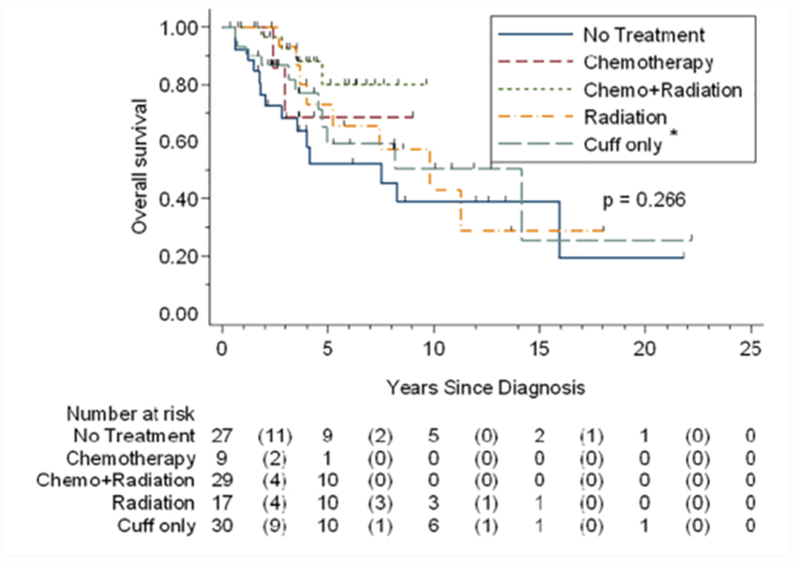
*Cuff = brachytherapy; The median overall survival for the brachytherapy group was 15.15 months; radiation alone group was 9.8 months, and no treatment group was 7.5 months. The median overall survival was not reached for the chemotherapy alone or chemotherapy+ radiation groups. No significant differences in median survival were identified based on treatment type.
DISCUSSION
Our study provides long-term data on patients with a rare, pathologically confirmed, subtype of endometrial cancer. Although UCCC is aggressive, our early stage patients have an OS approaching 10 years. Age ≥ 70 years was the only consistent clinicopathologic factor to negatively impact RFS and OS. Receipt of adjuvant treatment was not associated with improved survival, although the varied adjuvant therapy types were included together and small relative numbers of each distinct therapy make firm conclusions regarding the impact of adjuvant therapy problematic.
The association of advanced age with worse survival is consistent with the current literature [4, 11–16]. In our study, women ≥ 70 years had worse PFS and OS regardless of treatment modality. This finding encourages the consideration of quality of life implications and treatment toxicities when determining the utility of adjuvant treatment.
A small number of studies have unsuccessfully attempted to identify a subset of UCCC patients who would derive benefit from adjuvant therapy. Several studies have investigated the benefit of adjuvant radiation for UCCC. One of these studies investigated the impact of adjuvant radiation for early stage UCCC patients and failed to show a 5-year survival benefit (72% versus 77%, no radiation versus adjuvant radiation respectively, p=0.28) [13]. A retrospective review including 120 patients with any stage UCCC reported survival based on receipt of adjuvant radiation therapy, showing no appreciable difference between groups (77% versus 83%, surgery alone versus surgery and radiation, respectively, no p-value stated) [17]. Another study showed no additional benefit to adding brachytherapy to whole pelvic radiation for early stage UCCC [18]. Furthermore, a retrospective review including 38 women with UCCC reported the low propensity for abdominal failure, leading the authors to suggest avoiding whole pelvic radiation for these patients [19]. Subsequently, a retrospective review including patients with high risk endometrial cancer (n=37), of which approximately one-third had clear cell histology, examined the outcomes following adjuvant high-dose-rate brachytherapy and found 2-year vaginal control rates off 100% and DFS of 83% . In this study, adjuvant chemotherapy was not associated with improved survival outcomes [20]. Smaller studies have also failed to show a survival benefit from adjuvant radiation [21] or combination of chemotherapy and radiation [22]. Furthermore, a recent study using the National Cancer Database investigated outcomes for over 4,000 women with stage IA to IVA UCCC, and supported our findings, showing no difference in survival outcomes based on adjuvant treatment [11]. Taken together, these studies failed to identify a significant relationship between type/receipt of adjuvant treatment and RFS or OS.
Pure UCCC patients had worse survival on univariate analysis, but this relationship did not persist in the multivariate analysis. Pure UCCC were significantly less likely to receive adjuvant treatment compared to mixed UCCC, which may contribute to this finding. However, given that no survival differences were apparent based on receipt of any adjuvant treatment, an unrecognized confounding variable may account for our results. Additionally, our small sample size may have limited our ability to detect a difference in OS based on receipt of treatment.
Although not significant in our univariate analysis, the presence of lymphovascular space invasion correlated with worse survival in our multivariate analysis. Other studies that have investigated this association, yielding inconsistent results. One study including the review of 29 early stage UCCCs showed no association between LVSI and survival [6], while another study of 97 UCCC patients of all stages supported this association [23]. Another study of early stage UCCC correlated the presence of LVSI to improved survival [4], so the true extent of this relationship remains unknown.
Our study’s strengths include inclusion of a relatively large number of patients with stage I-II pathologically confirmed UCCC, followed over a long period of time. As this study is retrospective, inherent limitations do exist. The study is limited by the data available in the medical record and misclassification bias is possible. Additionally, selection bias may have influenced treatment decisions and potential confounding variables may not have been assessed. Furthermore, in order to obtain a large sample size, patient data were reviewed over the course of 16 years and treatment patterns/execution may have changed during that time.
Overall, our study adds to the current literature and reports the long-term outcomes for a large number UCCC patients. Providers should consider the quality of life impact on patients when making adjuvant treatment recommendations, especially for women who are older than 70 years of age.
HIGHLIGHTS.
Uterine clear cell patients are treated with a variety of adjuvant therapies and it is unclear if survival is improved Uterine clear cell patients over age 70 have worse RFS and OS regardless of treatment
Providers should consider quality of life when making adjuvant treatment recommendations, especially for women who are older than 70 years of age
HIGHLIGHTS.
Adjuvant therapy is not associated with survival for early stage UCCC
Uterine clear cell patients over age 70 have worse RFS and OS
Lymph node assessment was not associated with improved OS
Acknowledgments
FUNDING
Supported by the NIH/NCI under award numbers 5T32 CA101642, NIH 2P50 CA098258-06, NIH P30CA016672, and Andrew Sabin Family Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
None of the authors have any relevant financial interests or conflicts to disclose.
REFERENCES
- [1].Olawaiye AB, Boruta DM 2nd Management of women with clear cell endometrial cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;113(2):277–83. [DOI] [PubMed] [Google Scholar]
- [2].Mendivil A, Schuler KM, Gehrig PA. Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes. Cancer control : journal of the Moffitt Cancer Center. 2009;16(1):46–52. [DOI] [PubMed] [Google Scholar]
- [3].Webb GA, Lagios MD. Clear cell carcinoma of the endometrium. Am J Obstet Gynecol. 1987;156(6):1486–91. [DOI] [PubMed] [Google Scholar]
- [4].Cirisano FD Jr., Robboy SJ, Dodge RK, Bentley RC, Krigman HR, Synan IS, et al. Epidemiologic and surgicopathologic findings of papillary serous and clear cell endometrial cancers when compared to endometrioid carcinoma. Gynecol Oncol. 1999;74(3):385–94. [DOI] [PubMed] [Google Scholar]
- [5].Thomas M, Mariani A, Wright JD, Madarek EO, Powell MA, Mutch DG, et al. Surgical management and adjuvant therapy for patients with uterine clear cell carcinoma: a multi-institutional review. Gynecol Oncol. 2008;108(2):293–7. [DOI] [PubMed] [Google Scholar]
- [6].Carcangiu ML, Chambers JT. Early pathologic stage clear cell carcinoma and uterine papillary serous carcinoma of the endometrium: Comparison of clinicopathologic features and survival. International Journal of Gynecological Pathology. 1995;14(1):30–8. [DOI] [PubMed] [Google Scholar]
- [7].Malpica A, Tornos C, Burke TW, Silva EG. Low-stage clear-cell carcinoma of the endometrium. American Journal of Surgical Pathology. 1995;19(7):769–74. [DOI] [PubMed] [Google Scholar]
- [8].NCCN clincial practice guidelines in oncology. Uterine neoplasms version 2.2018.
- [9].Lindahl B, Persson J, Ranstam J, Willén R. Long-term survival in uterine clear cell carcinoma and uterine papillary serous carcinoma. Anticancer Research. 2010;30(9):3727–30. [PubMed] [Google Scholar]
- [10].Harris PA T R, Thielke R, Payne J, Gonzalez N, Conde JG,. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nieto K, Adams W, Pham N, Block AM, Grover S, Small W Jr., et al. Adjuvant therapy in patients with clear cell endometrial carcinoma: An analysis of the National Cancer Database. Gynecol Oncol. 2018;148(1):147–53. [DOI] [PubMed] [Google Scholar]
- [12].Abdulfatah E, Sakr S, Thomas S, Al-Wahab Z, Mutch DG, Dowdy S, et al. Clear Cell Carcinoma of the Endometrium: Evaluation of Prognostic Parameters in a Multi-institutional Cohort of 165 Cases. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2017;27(8):1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim A, Schreiber D, Rineer J, Choi K, Rotman M. Impact of adjuvant external-beam radiation therapy in early-stage uterine papillary serous and clear cell carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e639–e44. [DOI] [PubMed] [Google Scholar]
- [14].Chang-Halpenny CN, Natarajan S, Hwang-Graziano JM. Early-stage Uterine Pure and Mixed Clear Cell Carcinoma. American Journal of Clinical Oncology: Cancer Clinical Trials. 2018;41(4):371–8. [DOI] [PubMed] [Google Scholar]
- [15].Christopherson WM, Alberhasky RC, Connelly PJ. Carcinoma of the endometrium: I. A clinicopathologic study of clear- cell carcinoma and secretory carcinoma. Cancer. 1982;49(8):1511–23. [DOI] [PubMed] [Google Scholar]
- [16].Martinez AA, Weiner S, Podratz K, Armin AR, Stromberg JS, Stanhope R, et al. Improved outcome at 10 years for serous-papillary/clear cell or high-risk endometrial cancer patients treated by adjuvant high-dose whole abdomino-pelvic irradiation. Gynecol Oncol. 2003;90(3):537–46. [DOI] [PubMed] [Google Scholar]
- [17].Creasman WT, Kohler MF, Odicino F, Maisonneuve P, Boyle P. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004;95(3):593–6. [DOI] [PubMed] [Google Scholar]
- [18].Nagar H, Yan W, Parashar B, Nori D, Chao KSC, Christos P, et al. Adjuvant Pelvic Radiation Therapy ± Vaginal Brachytherapy in Patients With High-risk Stage I or Stage II Uterine Papillary Serous, Clear Cell, and High-grade Endometrioid Carcinoma. American Journal of Clinical Oncology: Cancer Clinical Trials. 2016;39(4):335–9. [DOI] [PubMed] [Google Scholar]
- [19].Murphy KT, Rotmensch J, Yamada SD, Mundt AJ. Outcome and patterns of failure in pathologic stages I-IV clear-cell carcinoma of the endometrium: implications for adjuvant radiation therapy. International journal of radiation oncology, biology, physics. 2003;55(5):1272–6. [DOI] [PubMed] [Google Scholar]
- [20].Townamchai K, Berkowitz R, Bhagwat M, Damato AL, Friesen S, Lee LJ, et al. Vaginal brachytherapy for early stage uterine papillary serous and clear cell endometrial cancer. Gynecol Oncol. 2013;129(1):18–21. [DOI] [PubMed] [Google Scholar]
- [21].Rauh-Hain JA, Costaaggini I, Olawaiye AB, Growdon WB, Horowitz NS, Del Carmen MG. A comparison of outcome in patients with Stage 1 clear cell and grade 3 endometrioid adenocarcinoma of the endometrium with and without adjuvant therapy. EUR J GYNAECOL ONCOL. 2010;31(3):284–7. [PubMed] [Google Scholar]
- [22].Foerster R, Kluck R, Rief H, Rieken S, Debus J, Lindel K. Survival of women with clear cell and papillary serous endometrial cancer after adjuvant radiotherapy. Radiation Oncology (London, England). 2014;9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Abeler VM, Kjørstad KE. Clear cell carcinoma of the endometrium: A histopathological and clinical study of 97 cases. Gynecol Oncol. 1990;40(3):207–17. [DOI] [PubMed] [Google Scholar]


