Figure 9. Dual PTMs may function as dueling PTMs.
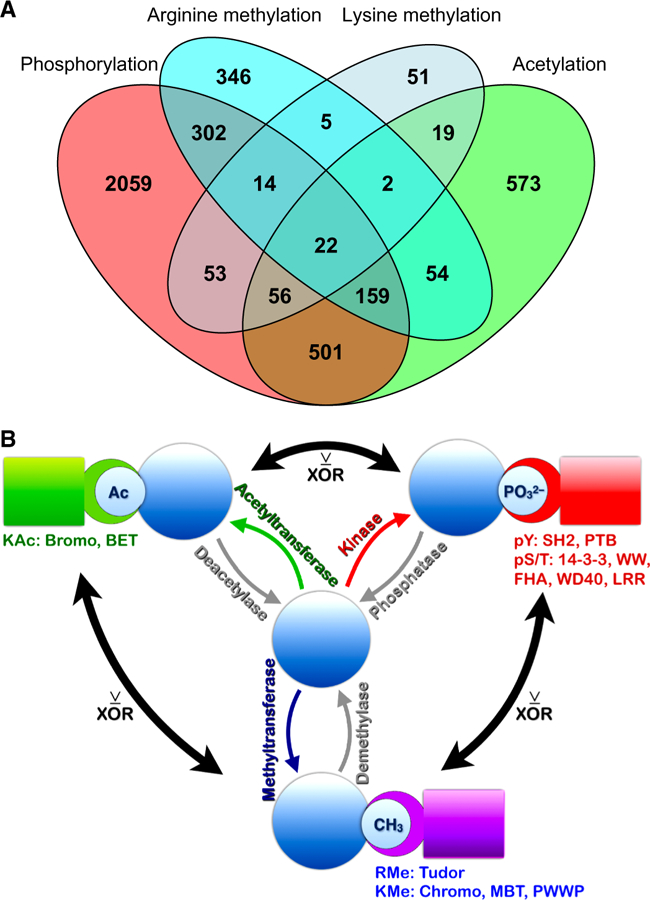
(A) Venn diagram showing the overlap among proteins modified by more than one type of PTM. A similar Venn diagram in which tyrosine and serine/threonine phosphorylation are separated is shown in fig. S15A. (B) Schematic of the findings. Proteins in molecular signaling pathways modified by more than one PTM have different sets of interacting proteins (5, 6). PTM-driven interactions occur through recognition of specifically modified amino acid residues by protein domains listed under the PTM type in the figure. Acetylated (Ac) proteins interact with bromodomain and BET (Bromodomain and extraterminal domain) proteins (green); methylated proteins interact with proteins containing Tudor domains if methylated on arginine (RMe); or Chromo, PWWP (‘Pro-Trp-Trp-Pro’), and MBT (malignant brain tumor) domains if methylated on lysine (KMe) (purple). Phosphorylated proteins interact with several protein families (red): tyrosine phosphorylated proteins (pY) interact with SH2 (SRC-homology domain 2) and PTB (phosphotyrosine binding) domains; serine/threonine phosphorylated proteins (pS/T) interact with a variety of proteins including 14–3-3 protein family members, and proteins containing the domains WW (domain with 2 conserved Trp residues), FHA (forkhead-associated domain), WD40 (WD or beta-transducin repeats), and LRR (leucine-rich repeats). Our data suggest that where inverse correlations exist between different PTMs, these may function as exclusive “OR” (XOR) switches to direct alternative cellular outcomes. This principle applies to phosphorylation vs. acetylation; phosphorylation vs. methylation; and methylation vs. acetylation.
