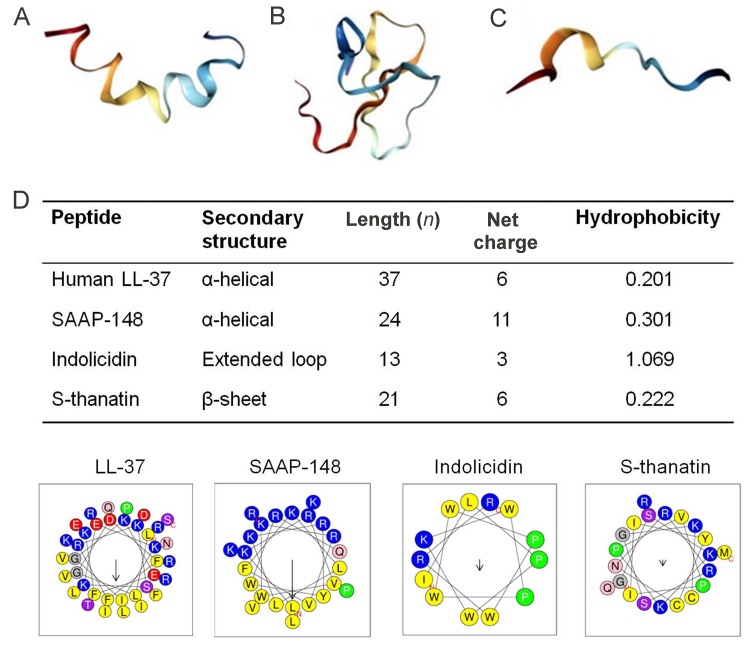
Figure 2. Structural diversity and helical wheel projections of representative AMPs.
A: α-helical-magainin (PDB ID 2LSA). B: β-sheet-chicken ovo-defensin (PDB ID 2MJK). C: Extended coil-tritrpticin. Images were created with Protein Data Bank (PDP) (Bioinformaticsdoi:10.1093/bioinformatics/bty419) (Rose et al., 2018) and visualized with Jmol software. D: Helical wheel projections of four representative peptides showing physical properties canonical to all AMPs, including distribution of amino acid residues, net charge, and hydrophobicity established to correlate with antimicrobial activity, selectivity, and cytotoxicity. Positively charged residues (polar) are represented as blue circles and hydrophobic (nonpolar) residues are yellow circles. Wheels projections, net charge, and hydrophobicity of AMPs were generated with HeliQuest webserver (http://heliquest.ipmc. cnrs.fr/).