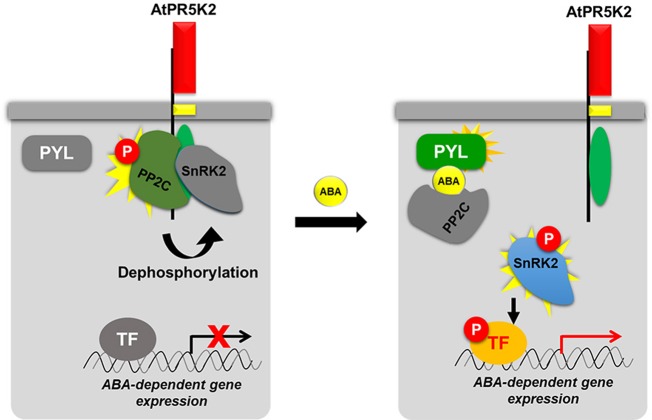
Figure 7.
Proposed working model of AtPR5K2 in ABA core signaling. AtPR5K2 is single transmembrane protein with an N-terminal thaumatin-like domain (red box), a single transmembrane domain (yellow box), and a C-terminal Ser/Thr kinase domain (green oval). In the absence of ABA, the phosphatase activity of the PP2Cs is enhanced by AtPR5K2-dependent phosphorylation. The activated PP2Cs dephosphorylate the SnRK2s, decreasing their activity. Thus, the ABA-dependent gene expression regulated by SnRK2 is attenuated. In the presence of ABA, the protein–protein interaction affinities among AtPR5K2, PP2Cs, and SnRK2s are weakened. This means that the PP2Cs and SnRK2 are released from AtPR5K2, freeing SnRK2.6 to autophosphorylate itself and phosphorylate the transcription factors regulating ABA-dependent gene expression. In the case of the PP2Cs and SnRK2.6, a vivid coloration indicates an active status, while the gray color indicates an inactive status. The red circle containing the letter “P” indicates phosphorylation.