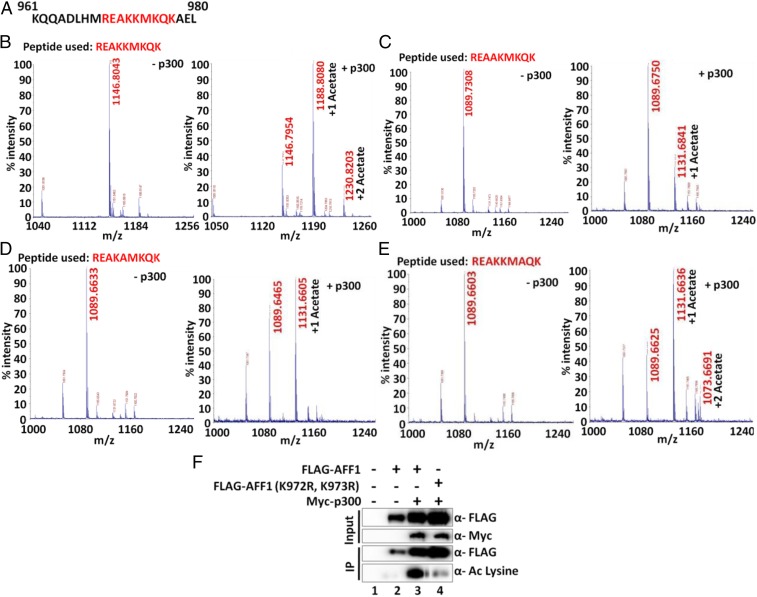
Fig. 3.
Mass spectrometric analysis of p300-mediated in vitro acetylation of AFF1 using peptides from the 969 to 977 region. (A) Amino acid sequence of AFF1 961 to 980. Peptide sequences used in the in vitro acetylation assay are indicated in red. (B) Mass spectrometric analysis of p300-dependent acetylation of WT AFF1 peptide. Two peaks with masses corresponding to mono- and di-acetylation events are observed. (C) Mass spectrometric analysis of p300-dependent acetylation of mutant (K972A) AFF1 peptide. A single peak with a mass corresponding to a monoacetylation event is observed. (D) Mass spectrometric analysis of p300-dependent acetylation of mutant (K973A) AFF1 peptide. A single peak with a mass corresponding to a monoacetylation event is observed. (E) Mass spectrometric analysis of p300-dependent acetylation of mutant (K975A) AFF1 peptide. Two peaks with masses corresponding to monoacetylation and diacetylation events are observed. (F) Analysis of WT and mutant (K972R, K973R) AFF1 acetylation by p300 in 293T cells. Cells were transfected with indicated plasmids, and anti-FLAG immunoprecipitated samples were analyzed by immunoblot using pan-acetyl lysine-specific antibody.