Abstract
Objective
Depression is linked to excess weight, insulin resistance, and type 2 diabetes (T2D). We previously reported that in adolescent girls at-risk for T2D with moderately elevated depression, randomization to cognitive-behavioral therapy (CBT) produced greater decreases in depression at post-treament and greater decreases in fasting/2-h insulin at 1 year, compared to health education (HE). The current study is a secondary analysis of this parallel-group randomized controlled trial. We examined whether decreasing depression explained intervention effects on body composition and insulin outcomes. We hypothesized that decreases in depression would be an explanatory mediator and that indirect effects would be strongest at higher levels of baseline depression.
Methods
Participants were 12–17 years girls with overweight/obesity and family history of T2D randomized to 6-week group CBT (n = 58) or HE (n = 61). Procedures took place at an outpatient pediatric clinic. At baseline, post-treatment, and 1 year, adolescents completed the Center for Epidemiologic Studies-Depression Scale to assess depression symptoms; body mass index (BMI [kg/m2]) was measured from height/fasting weight; insulin resistance was derived from 2-h oral glucose testing. Adiposity was assessed with dual-energy X-ray absorptiometry at baseline and 1 year. Indirect effects of intervention were tested on 1-year changes in BMI, adiposity, and insulin through decreases in depression. Baseline depression was tested as a moderator of mediation.
Results
There was an indirect effect of CBT on decreased 1-year fasting insulin via decreases in depression during treatment, among adolescents with more elevated baseline depression.
Conclusions
Decreasing elevated depression may be one mechanism in the targeted prevention of T2D in at-risk adolescents.
Keywords: clinical trial, cognitive-behavioral therapy, depression, diabetes, obesity
Introduction
Obesity in adolescents is associated with heightened risk of developing type 2 diabetes (T2D) (Pulgaron & Delamater, 2014). One psychosocial correlate of both obesity and T2D is depression; adolescent depression symptoms are related concurrently and prospectively to excess weight gain, onset of obesity, worsening insulin resistance, and onset of T2D (Hannon, Rofey, Lee, & Arslanian, 2013; Markowitz, Friedman, & Arent, 2008; Nemiary, Shim, Mattox, & Holden, 2012). While depression symptoms partially may affect T2D risk via their impact on adiposity, depression symptoms also have been posited to exert a unique effect on insulin resistance through stress-related behavioral and physiological mechanisms, including emotional eating, physical inactivity, sleep disruption, dysregulation of the hypothalamic-pituitary-adrenal axis, and/or chronic inflammation (Markowitz et al., 2008).
Most research investigating adolescent interventions to prevent T2D has focused on intensive lifestyle interventions, including nutrition education and physical activity several times per week for a prolonged period (e.g., 6 months), to achieve weight loss (Shaw et al., 2009). These approaches are burdensome and cost ineffective and, unfortunately, have demonstrated limited effectiveness in adolescents (Gonzalez, Tanenbaum, & Commissariat, 2016). In light of possible putative mechanisms linking elevated depression symptoms to T2D, psychosocial interventions designed to target depression symptoms in adolescents may help to ameliorate excess adiposity and insulin resistance as precursors to T2D. Pinpointing to what extent decreasing depression symptoms improves weight and insulin outcomes is a crucial next step in elucidating potential mechanisms of change as part of a precision medicine approach to addressing obesity and preventing T2D (Kelly, Marcus, Yanovski, Yanovski, & Osganian, 2018).
To this end, the current study is a secondary data analysis of a parallel-group randomized controlled trial comparing a brief cognitive-behavioral therapy (CBT) group depression intervention to a health education (HE) control group for preventing worsening insulin resistance in adolescents with heterogeneous, mild-to-moderate depression symptoms. Main outcomes have been reported (Shomaker et al., 2016, 2017), where CBT had no statistically significant effects on depression or insulin outcomes. However, among adolescents with moderately elevated depression symptoms at baseline, those randomized to CBT experienced a greater reduction in depression at post-treatment compared to adolescents in HE (Shomaker et al., 2016). At 1 year, adolescents randomized to CBT had lower 2-h insulin from baseline, and less fasting and 2-h insulin from post-treatment, as compared to HE (Shomaker et al., 2017).
The current study sought to integrate these main outcomes by testing mediation models to evaluate decreases in depression symptoms during the intervention as an intervening variable to explain the effects of CBT, versus HE, on subsequent post-treatment to 1-year changes in body mass index (BMI), adiposity, and insulin resistance. There is growing support for testing mediation models to assess the mechanisms of change by which interventions influence outcomes (O'Rourke & MacKinnon, 2018). Evidence of indirect effects, even in the absence of overall intervention effects, may provide valuable information about how change in depression might operate as a mechanism of change by which CBT helps to lessen T2D risk. We hypothesized that there would be significant indirect effects of intervention on changes in each of these outcomes through the planned intervention target: decreases in depression symptoms during the intervention. Further, based upon the notion that the depression-insulin resistance connection is not entirely explained by these factors’ shared association with obesity, we hypothesized that indirect effects of condition on insulin outcomes through decreased depression would exist even after controlling for initial adiposity and changes in adiposity. Finally, we expected that the indirect effects would be moderated by baseline depression symptoms, in light of improvements in depression and insulin outcomes after CBT versus HE only in those who reported more elevated depression symptoms at baseline (Shomaker et al., 2016, 2017).
Method
Details regarding research design and methods have been reported (Shomaker et al., 2016, 2017). Key methods are described here.
Participants
Participants were adolescent girls 12–17 years (N = 119). Inclusion criteria for the randomized controlled trial were: female; overweight (BMI ≥85th percentile); family history of T2D, prediabetes, or gestational diabetes in ≥1 first- or second-degree relative; and moderately elevated depression symptoms indicated by a total score ≥16 on the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977). Exclusion criteria included: current psychiatric symptoms that necessitated treatment (e.g., major depressive disorder); major medical problem (e.g., T2D, fasting glucose >126 mg/dl or 2-h glucose >200 mg/dl); medication affecting insulin, weight, or mood (e.g., antidepressants); recent significant weight loss; participation in structured weight loss intervention or psychotherapy; and pregnancy. Procedures took place in an outpatient pediatric clinic at the NIH Clinical Research Center in Bethesda, Maryland. The Eunice Kennedy Shriver NICHD Institutional Review Board approved all procedures. Parents/caregivers provided informed written consent. Adolescents provided informed written assent. Adolescents were compensated for participation.
Procedures
Recruitment was initiated in September 2011 and concluded in May 2014. Nine hundred thirty-seven families contacted the study team; 178 (19%) completed a screening visit and 119 (67%) of adolescents that completed a screening visit were randomized (Shomaker et al., 2016). Measures of demographics, anthropometrics (including BMI and adiposity), depression, and insulin were collected at baseline to determine eligibility and evaluate pre-treatment functioning. Eligible adolescents were randomized to either a CBT or HE group program (1: 1 allocation) that met for 1-h sessions once per week for 6 weeks. Randomization was stratified by age and race/ethnicity, and randomization strings were generated by an electronic program with permuted blocks (Shomaker et al., 2016). After determining eligibility, the study coordinator assigned participants to interventions. Follow-ups were initiated in February 2012 and concluded by July 2015. Of those randomized to intervention, 108 (91%) completed a post-treatment assessment and 87 (73%) completed a 1-year assessment, with no differences by condition (Shomaker et al., 2016, 2017). Assessors at baseline and follow-up were blind to allocation. BMI, depression, and insulin were re-assessed at post-treatment. BMI, adiposity, depression, and insulin were re-assessed again at 1 year.
Measures
Medical Information
At baseline, an endocrinologist or nurse practitioner conducted a medical history, including family history of T2D, and a physical exam. Breast Tanner staging was assessed by physical inspection and palpitation (Marshall & Tanner, 1969).
Anthropometrics
At all intervals, weight was obtained by digital scale in a fasting state with shoes and outer clothing removed. Height was averaged from three measurements with a wall stadiometer. BMI (kg/m2) was calculated and standard scores were estimated from CDC 2000 standards (Ogden et al., 2002). For primary analyses, we evaluated change in raw BMI over time, as recommended (Ogden et al., 2002). At baseline and 1 year, percentage adiposity (fat mass/total mass) was derived from dual-energy X-ray absorptiometry (iDXA, GE Healthcare, Madison, WI, USA); change in percentage adiposity was also examined.
Depression Symptoms
The 20-item CES-D (Radloff, 1977) was administered at all intervals. A total score, ranging from 0 to 60, is generated by summing responses across all items; higher scores reflect higher depression symptoms. The CES-D has been shown to be reliable and valid in samples of adolescents, and confirmatory factor analysis suggests that the standard scoring of the CES-D with a total score is justified in adolescents (e.g., Phillips et al., 2006). In the current study, the CES-D showed good internal reliability at baseline (α = .80), post-treatment (α = .80), and 1 year (α = .86).
Oral Glucose Tolerance Testing
At all intervals, an oral glucose tolerance test (OGTT) was administered to ascertain fasting insulin, 2-h insulin, whole body insulin sensitivity, and homeostasis model assessment of insulin resistance. The OGTT was conducted in the morning following a 10-h overnight fast. Participants received 1.75 g/kg of dextrose (maximum 75 g). Blood was sampled for serum insulin and glucose at fasting and 30, 60, 90, and 120 min after dextrose as previously reported (Shomaker et al., 2016, 2017).
Interventions
The intervention groups have been described previously (Shomaker et al., 2016, 2017). CBT was a manualized depression prevention group program selected because it has demonstrated efficacy for decreasing depression symptoms and reducing incidence of major depressive disorder, particularly in adolescents with moderately elevated depression symptoms (Stice, Rohde, Gau, & Wade, 2010; Stice, Rohde, Seeley, & Gau, 2008). CBT includes psychoeducation and activities on the interconnectedness of feelings, thoughts, and behaviors, self-monitoring, cognitive restructuring, increasing pleasant activities and rewards, and planning ahead to cope with daily hassles and major stressful life events (Stice et al., 2008). The median of expert ratings of CBT facilitator competence was 7.8 (1 = poor to 10 = superior) and fidelity was 7.7 (1 = none to 10 = perfect match) (Shomaker et al., 2016).
HE was a manualized, attention-matched control group adapted from a didactic middle and high school health curriculum (Bravender, 2005). HE includes presentations, handouts, and videos on topics including alcohol and drug use, nutrition and body image, domestic violence, sun safety, exercise, and identifying depression and suicide risk (Bravender, 2005). Median expert ratings of HE sessions for overlap with CBT content was 1.0 (1 = none to 10 = perfect match), confirming independence between interventions (Shomaker et al., 2016).
CBT and HE groups ranged in size from 4 to 8 adolescents. The groups met during nonschool hours and were cofacilitated by two individuals, including psychologists, postdoctoral fellows, and/or doctoral-level graduate students.
Data Analysis
Data were analyzed using SPSS for Windows, version 25 (IBM Corporation, Armonk, NY, USA) and significance was determined as p < .05. Single imputation using Expectation-Maximization method in SPSS was used to handle missing data (14% of all data points). Following current guidelines for evaluating intervening variables, indirect effects were tested using Hayes’ (v 3.0) PROCESS SPSS macro (Hayes, 2018). Five thousand bootstrap resamples were collected to generate a bias-corrected 95% confidence interval (CI; Preacher & Hayes, 2004).
First, we tested simple mediational models to determine whether change in depression symptoms from baseline to post-treatment was an intervening variable in the relationship between intervention assignment (CBT vs. HE) and change in outcome from post-treatment to 1 year. Outcome measures included changes in BMI, percent adiposity, insulin sensitivity, insulin resistance, fasting, and 2-h insulin. Second, we tested moderated mediation models to examine whether baseline depression symptoms moderated the relationship between intervention and change in depression, as well as whether the strength of the indirect effect was conditional on the level of baseline depression. The rationale for moderated mediation was based on our finding of greater decreases in depression symptoms in CBT versus HE from baseline to post-treatment (Shomaker et al., 2016). Moreover, previous studies have found support for greater effects of depression prevention interventions among individuals with elevated baseline depression symptoms (Stice, Shaw, Bohon, Marti, & Rohde, 2009).
Analyses were adjusted for baseline age, pubertal status, and degree of T2D family history, as well as the baseline and post-treatment level of the outcome change score. Analysis for percent adiposity was adjusted for baseline, as opposed to post-treatment percent adiposity because the latter was not obtained. Analyses for insulin sensitivity, insulin resistance, fasting insulin, and 2-h insulin were also adjusted for baseline percent adiposity and 1-year change in percent adiposity.
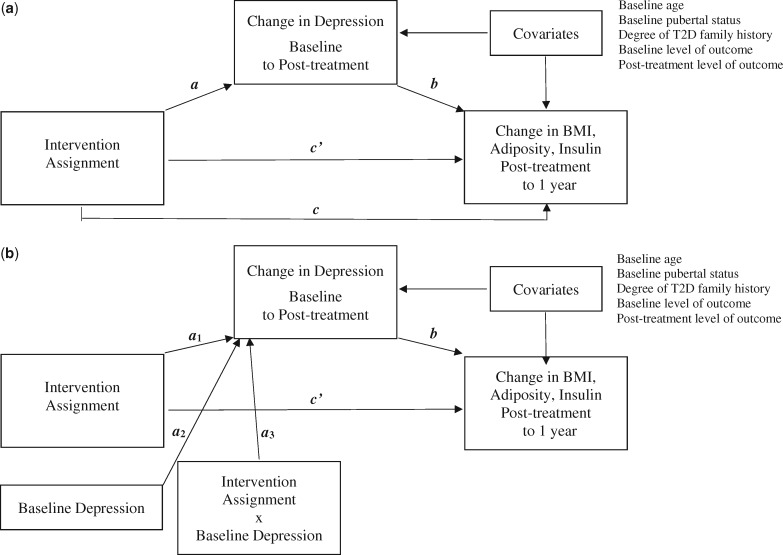
We examined the following pathways in each of the models (Hayes, 2009) (Figure 1a). The a1-path represents the effect of the intervention on change in depression symptoms from baseline to post-treatment. The b-path is the effect of change in depression symptoms on change in the dependent variable from post-treatment to 1 year. The indirect effect, ab-path, is the portion of the total effect of intervention on the dependent variable that occurs through change in depression. The direct effect, c’-path, reflects the effect of the intervention on the dependent variable that is independent of change in depression. Finally, the total effect, c-path, is the overall effect of the intervention on the outcome, combining direct and indirect effects. In the moderated mediation models (Figure 1b), the a3-path represents the moderation of the a1-path by baseline level of depression symptoms. The conditional indirect effect (a3b-path) tests whether the ab-path is conditional on the interaction between intervention and baseline depression. In line with current recommendations for testing and interpreting mediational models (Hayes, 2009), we reported results of all pathways, even in the absence of a significant total effect. Moreover, we reported significant conditional effects, even in the absence of significant a3- or a3b-paths. Hayes (2015) suggests that it is useful to report this information regarding when an indirect effect is significant at certain levels of the moderator (Hayes, 2015).
Figure 1.
Path diagrams of (a) the simple mediation model and (b) the moderated mediation model.
Results
Baseline descriptive statistics are displayed in Table I. A summary of the simple mediation models is shown in Table II. A summary of the moderated mediation models is shown in Table III.
Table I.
Descriptive Baseline Information by Intervention Assignment
| Characteristic | CBT (n = 61) | HE (n = 58) | p value |
|---|---|---|---|
| Age (years), mean (SD) | 14.98 (1.58) | 15.05 (1.55) | 0.81 |
| Race, n (%) | 0.69 | ||
| Non-Hispanic Black | 39 (63.9) | 35 (60.3) | |
| Non-Hispanic White | 8 (13.1) | 11 (19.0) | |
| Hispanic | 7 (11.5) | 5 (10.3) | |
| Other | 7 (11.5) | 6 (10.3) | |
| Family history of type 2 diabetes, n (%) | 0.13 | ||
| First-degree relative | 20 (32.8) | 27 (46.6) | |
| Second-degree relative | 41 (67.2) | 31 (53.4) | |
| Tanner breast pubertal stage, n (%) | 0.44 | ||
| 1–2 | 2 (3.3) | 2 (3.4) | |
| 3 | 7 (11.4) | 4 (6.9) | |
| 4 | 7 (11.5) | 13 (22.4) | |
| 5 | 45 (73.8) | 39 (67.2) | |
| Depression symptoms, mean (SD) | 25.31 (7.25) | 24.49 (7.45) | 0.54 |
| BMI (kg/m2), mean (SD) | 31.71 (6.05) | 34.26 (6.91) | 0.03 |
| BMI (z score), mean (SD) | 1.89 (0.45) | 2.05 (0.48) | 0.05 |
| Elevated BMI z > 1.00, n (%) | 61 (100) | 58 (100) | 0.99 |
| % Adiposity, mean (SD) | 40.83 (5.38) | 42.54 (6.04) | 0.10 |
| Elevated % adiposity (≥36.7%), n (%) | 47 (77.0) | 49 (84.5) | 0.31 |
| WBISI, mean (SD) | 2.70 (1.47) | 2.34 (1.40) | 0.17 |
| HOMA-IR, mean (SD) | 4.93 (2.92) | 5.80 (3.30) | 0.13 |
| Elevated insulin resistance (≥3.16), n (%) | 43 (70.5) | 45 (77.6) | 0.38 |
| Fasting insulin (µIU/ml), mean (SD) | 22.24 (12.37) | 26.38 (14.77) | 0.10 |
| Hyperinsulinemia (≥15 µIU/ml), n (%) | 41 (67.2) | 46 (79.3) | 0.14 |
| Two-hour insulin (µIU/ml), mean (SD) | 115.49 (81.54) | 134.50 (100.86) | 0.26 |
Note. CBT = cognitive-behavioral group; HE = health education group; BMI = body mass index; HOMA-IR = homeostasis model assessment of insulin resistance, with higher values representing worse insulin resistance and lower values little to no insulin resistance; WBISI = whole body insulin sensitivity index, with higher values reflecting better insulin sensitivity and lower values poorer insulin sensitivity; SD = standard deviation.
Table II.
Simple Mediation Analyses Testing Change in Depression Symptoms During the Intervention Period as an Intervening Variable Underlying Intervention’s Effects on Body Composition and Insulin Outcomes
| Total effect (c) | Path a | Path b | Indirect effect (ab) | Direct effect (c’) | |
|---|---|---|---|---|---|
| Outcome | B, SE (95% CI) | B, SE (95% CI) | B, SE (95% CI) | B (95% CI) | B, SE (95% CI) |
| BMI (kg/m2) | −0.43, 0.29 (−0.99, 0.14) | −2.42, 1.38 (−5.15, 0.31) | 0.05, 0.02 (0.01, 0.09) | −0.12 (−0.34, 0.01) | −0.30, 0.28 (−0.86, 0.26) |
| %Adiposity | 0.00, 0.00 (−0.01, 0.01) | −2.19, 1.38 (−4.91, 0.54) | 0.001, 0.00 (0.0003, 0.002) | 0.00 (−0.01, 0.00) | 0.00, 0.01 (−0.01, 0.01) |
| WBISI | 0.08. 0.18 (−0.27, 0.44) | −2.30, 1.33 (−4.94, 0.35) | −0.03, 0.01 (−0.05, −0.0003) | 0.06 (−0.01, 0.18) | 0.03, 0.18 (−0.33, 0.38) |
| HOMA-IR | −0.01, 0.41 (−0.82, 0.79) | −2.23, 1.34 (−4.90, 0.43) | 0.06, 0.03 (0.01, 0.12) | −0.14 (−0.44, 0.02) | 0.12, 0.40 (−0.68, 0.92) |
| Fasting insulin | −0.34, 1.71 (−3.73, 3.04) | −2.20, 1.35 (−4.87, 0.47) | 0.28, 0.12 (0.04, 0.51) | −0.61 (−1.87, 0.12) | 0.27, 1.69 (− 3.09, 3.63) |
| Two-hour insulin | −1.58, 10.54 (−22.47, 19.31) | −2.52, 1.38 (−5.25, 0.22) | −0.13, 0.73 (−1.58, 1.32) | 0. (−4.43, 4.66) | −1.90. 10.75 (−23.20, 19.40) |
Note. BMI = body mass index; HOMA-IR = homeostasis model assessment of insulin resistance, with higher values representing greater insulin resistance and lower values little to no insulin resistance; WBISI = whole body insulin sensitivity index, with higher values reflecting better insulin sensitivity and lower values poorer insulin sensitivity. Path c indicates the total effect from intervention to outcome; Path a indicates the directional path from intervention to the mediator; Path b indicates the directional path from the mediator to the outcome; Path ab indicates the indirect path from intervention to outcome indirectly through the mediator; Path c’ indicates the direct path from intervention to outcome independently of the mediator. Significant paths are presented in bolded font.
Table III.
Moderated Mediation Analyses Testing Change in Depression Symptoms During the Intervention Period as an Intervening Variable Underlying Intervention’s Effects on Body Composition and Insulin Outcomes, Moderated by Baseline Depression Symptoms
| Path a | Moderation of Path a (a3) | Path b | Index of moderated mediation (a3b) | Direct effect (c’) | |
|---|---|---|---|---|---|
| Outcome | B, SE (95% CI) | ΔR2 (p) | B, SE (95% CI) | B (95% CI) | B, SE (95% CI) |
| BMI (kg/m2) | 4.37, 3.76 (−3.08, −11.84) | 0.01 (0.10) | 0.05, 0.02 (0.01, 0.09) | −0.01 (−0.03, 0.00) | −0.30, 0.28 (−0.86, 0.26) |
| %Adiposity | −4.59, 3.73 (−2.80, −11.98) | 0.01 (0.08) | 0.001, 0.01 (0.0003, 0.002) | 0.00 (0.00, 0.00) | 0.00, 0.01 (−0.01, 0.01) |
| WBISI | 5.65, 3.66 (−1.62, −12.91) | 0.02 (0.04) | −0.03, 0.12 (−0.05, -0.0003) | 0.01 (0.00, 0.02) | 0.03, 0.18 (−0.33, 0.38) |
| HOMA-IR | 5.70, 3.72 (−1.67, −13.06) | 0.02 (0.04) | 0.06, 0.03 (0.01, 0.12) | −0.02 (−0.06, 0.00) | 0.12, 0.40 (−0.68, 0.92) |
| Fasting insulin | 5.64, 3.72 (−1.73, −13.01) | 0.02 (0.046) | 0.28, 0.12 (0.04, 0.51) | −0.08 (−0.24, −0.002) | 0.27, 1.69 (−3.09, 3.63) |
| Two-hour insulin | 5.30, 3.70 (−2.03, −12.64) | 0.02 (0.045) | −0.13. 0.73 (−1.58, 1.32) | 0.04 (−0.57, 0.46) | −1.90, 10.75 (−23.20, −19.40) |
Note. BMI = body mass index; HOMA-IR = homeostasis model assessment of insulin resistance, with higher values representing greater insulin resistance and lower values little to no insulin resistance; WBISI = whole body insulin sensitivity index, with higher values reflecting better insulin sensitivity and lower values poorer insulin sensitivity. Path c indicates the total effect from intervention to outcome; Path a indicates the directional path from intervention to the mediator; Path b indicates the directional path from the mediator to the outcome; Path ab indicates the indirect path from intervention to outcome indirectly through the mediator; Path c’ indicates the direct path from intervention to outcome independently of the mediator. Significant paths are presented in bolded font.
BMI
In the simple mediation model, intervention (CBT versus HE) did not have an effect on the change in 1-year BMI (c-path) and there was no effect of intervention on changes in depression (a-path). However, a greater decrease in depression was associated with a greater decrease in BMI [b-path: B = 0.05, SE = 0.02, CI = (0.01, 0.09)]. The indirect effect (ab-path) and the direct path (c’-path) were not significant. In the moderated mediation model, CBT was associated with a significant decrease in depression among participants with elevated baseline depression [conditional a-path at + 1 SD: B = −3.29, SE = 1.54, 95% CI = (−6.34, −0.24)]; however, the interaction did not reach significance (a3-path), indicating that effect of intervention on change in depression did not significantly differ depending on baseline depression. Decrease in depression was an explanatory mechanism by which CBT led to decrease in BMI among participants with elevated baseline depression [conditional ab-path at + 1 SD: B = −0.17, 95% CI (−0.46, −0.01); Table IV]; however, the index of moderated mediation was not significant (a3b-path), indicating that the indirect effect of CBT on BMI through decreases in depression was not significantly moderated by baseline depression.
Table IV.
Conditional Effects of the a1 and the ab Paths at Levels of Baseline Depression Symptoms (M ± SD)
| Conditional a3-path at M ± SD |
Conditional a3b-path at M ± SD |
|||||
|---|---|---|---|---|---|---|
| Outcome | B | SE | 95% CI | B | SE | 95% CI |
| BMI (kg/m2) | ||||||
| −1 SD | 0.20 | 1.52 | −2.81, 3.20 | 0.01 | 0.09 | −0.15, 0.21 |
| M | −1.55 | 1.09 | −3.72, 0.63 | −0.08 | 0.07 | −0.25, 0.04 |
| +1 SD | −3.29 | 1.54 | −6.34, −0.24 | −0.17 | 0.12 | −0.46, −0.01 |
| % Adiposity | ||||||
| −1 SD | 0.24 | 1.49 | −2.72, 3.19 | 0.00 | 0.00 | 0.00, 0.00 |
| M | −1.58 | 1.08 | −3.71, 0.56 | 0.00 | 0.00 | 0.00, 0.00 |
| +1 SD | −3.39 | 1.53 | −6.42, −0.37 | −0.0031 | 0.00 | −0.01, −0.0004 |
| WBISI | ||||||
| −1 SD | 0.45 | 1.47 | −2.46, 3.35 | −0.01 | 0.04 | −0.11, 0.08 |
| M | −1.72 | 1.06 | −3.82, 0.38 | 0.04 | 0.04 | −0.01, 0.15 |
| +1 SD | −3.89 | 1.50 | −6.87, −0.91 | 0.10 | 0.08 | 0.008, 0.29 |
| HOMA-IR | ||||||
| −1 SD | 0.54 | 1.49 | −2.41, 3.48 | 0.03 | 0.11 | −0.15, 0.28 |
| M | −1.61 | 1.08 | −3.75, 0.52 | −0.10 | 0.10 | −0.34, 0.04 |
| +1 SD | −3.76 | 1.53 | −6.79, −0.74 | −0.23 | 0.18 | −0.68, 0.00 |
| Fasting insulin | ||||||
| −1 SD | 0.55 | 1.49 | −2.41, 3.50 | 0.15 | 0.47 | −0.72, 1.20 |
| M | −1.58 | 1.08 | −3.71, 0.56 | −0.44 | 0.42 | −1.53, 0.13 |
| +1 SD | −3.70 | 1.52 | −6.72, −0.68 | −1.03 | 0.75 | −2.96, -0.08 |
| Two-hour insulin | ||||||
| −1 SD | 0.22 | 1.50 | −2.76, 3.20 | −0.03 | 1.17 | −2.28, 2.80 |
| M | −1.90 | 1.10 | −4.08, 0.27 | 0.25 | 1.69 | −3.71, 3.58 |
| +1 SD | −4.02 | 1.53 | −7.05, −0.99 | 0.52 | 3.36 | −7.40, 6.32 |
Note. BMI = body mass index; HOMA-IR = Homeostasis model assessment of insulin resistance, with higher values representing greater insulin resistance and lower values little to no insulin resistance; WBISI = whole body insulin sensitivity index, with higher values reflecting better insulin sensitivity and lower values poorer insulin sensitivity. The a3-path represents the moderation of the a1-path by baseline level of depression symptoms. The conditional indirect effect (a3b-path) tests whether the ab-path is conditional on the interaction between intervention and baseline depression. Significant paths are presented in bolded font.
Adiposity
In the simple mediation model, intervention did not have an effect on change in percent adiposity (c-path) and there was no effect of intervention on changes in depression (a-path). A greater decrease in depression was associated with a greater decrease in percent adiposity [b-path: B = 0.001, SE = 0.0003, CI = (0.0003, 0.002)]. The indirect effect (ab-path) and the direct path (c’-path) were not significant. In the moderated mediation model, CBT was associated with a significant decrease in depression among participants with elevated baseline depression [conditional a-path at +1 SD: B = −3.39, SE = 1.53, 95% CI = (−6.42, −0.37)]. However, the interaction was not significant (a3-path). Decrease in depression was an explanatory mechanism by which CBT versus HE led to decrease in percent adiposity among participants with elevated baseline depression (conditional ab-path at +1 SD: B = −0.003, 95% CI (−0.008, −0.0004)]; however, the index of moderated mediation was not significant (a3b-path).
Insulin Sensitivity
In the simple mediation model, intervention did not have an effect on change in 2-h insulin (c-path) and there was no effect of intervention on changes in depression (a-path). A greater decrease in depression was associated with a greater increase in insulin sensitivity [b-path: B = −0.03, SE = 0.01, CI = (−0.05, −0.0003)]. The indirect effect (ab-path) and the direct path (c’-path) were not significant. In the moderated mediation model, baseline depression moderated the effect of intervention on change in depression (a3-path: ΔR2 = .02, p < .05); participants with elevated baseline depression demonstrated a greater decrease in symptoms at post-treatment [conditional a-path at +1 SD: B = −3.89, SE = 1.50, 95% CI = (−6.87, −0.91)]. Decrease in depression was an explanatory mechanism by which CBT led to an increase in insulin sensitivity among participants with elevated baseline depression [conditional ab-path at +1 SD: B = 0.09, 95% CI (0.008, −0.29)]; however, the index of moderated mediation was not statistically significant (a3b-path).
Insulin Resistance
In the simple mediation model, intervention did not have an effect on change in insulin resistance (c-path) and there was no effect of intervention on changes in depression (a-path). A greater decrease in depression was associated with a greater decrease in insulin resistance [b-path: B = 0.06, SE = 0.03, CI = (0.01, 0.12)]. The indirect effect (ab-path) and the direct path (c’-path) were not significant. In the moderated mediation model, baseline depression moderated the effect of intervention on change in depression (a3-path: ΔR2 = .02, p < .05); participants with elevated baseline depression demonstrated a greater decrease in symptoms at post-treatment [conditional a-path at +1 SD: B = −3.76, SE = 1. 53, 95% CI = (−6.79, −0.74)]. The index of moderated mediation was not significant (a3b-path).
Fasting Insulin
In the simple mediation model, intervention did not have an effect on change in fasting insulin (c-path) and there was no effect of intervention on changes in depression (a-path). However, a greater decrease in depression was associated with a greater decrease in fasting insulin [b-path: B = 0.28, SE = 0.12, CI = (0.04, 0.51)]. The indirect effect (ab-path) and the direct path (c’-path) were not significant. In the moderated mediation model, baseline depression moderated the effect of intervention on change in depression (a3-path: ΔR2 = .02; p < .05); participants with elevated baseline depression demonstrated a greater decrease in symptoms at post-treatment [conditional a-path at + 1 SD: B = −3.70, SE = 1.52, 95% CI = (−6.72, −0.68)]. The index of moderated mediation was significant [β = −.08, 95% CI = (−0.24, −0.002)], meaning that CBT was associated with greater decreases in fasting insulin through decreases in depression as baseline depression increased. For every standard deviation increase in baseline depression symptoms, CBT decreased fasting insulin by 0.08 standard deviations, or almost 1 µIU/ml, through decreases in depression. The demonstrated effect size is somewhat small in magnitude, and thus may potentially limit the clinical significance of findings; however, it has been argued that significant effects that are small in magnitude can still communicate potentially useful information regarding putative mechanisms of change in mediation analysis (Agler & De Boeck, 2017).
Two-Hour Insulin
In the simple mediation model, intervention did not have an effect on change in 2-h insulin (c-path). There was no effect of intervention on change in depression (a-path), and change in depression was not associated with changes in 2-h insulin (b-path). The indirect effect (ab-path) and the direct path (c’-path) were not significant. In the moderated mediation model, baseline depression moderated the effect of intervention on change in depression (a3-path: R2 = .02, p < .05); participants with elevated baseline depression demonstrated a greater decrease in symptoms at post-treatment [conditional a-path at + 1 SD: B = −4.02, SE = 1.53, 95% CI = (−7.05, −1.00)]. The index of moderated mediation was not significant (a3b-path).
Discussion
The current study builds upon previously reported findings of a randomized trial comparing a short-term psychosocial intervention for depression to an attention-matched comparison group for prevention of worsening insulin resistance in adolescents (Shomaker et al., 2016, 2017). Preliminary findings suggested that CBT might offer psychological and metabolic benefits to youth with moderately elevated depression symptoms 1 year after the treatment (Shomaker et al., 2016, 2017). In the current study, we conducted a series of secondary mediational analyses to determine if decreases in depression explained the effects of intervention on subsequent improvements in BMI, adiposity, and insulin outcomes. We also tested moderated mediation to examine whether indirect effects were moderated by baseline depression symptoms.
We found a significant indirect effect of CBT, as compared to HE, on 1-year fasting insulin, even after accounting for baseline and 1-year change in adiposity. This indirect effect was significantly moderated by baseline depression level. CBT decreased depression symptoms among adolescents with more elevated baseline depression, and in turn, decreased depression was related to decreases in fasting insulin 1-year later. Although moderated mediation did not reach statistical significance for other outcomes, we did indeed find evidence of significant conditional indirect effects of CBT, as compared to HE, on 1-year BMI, adiposity, and insulin sensitivity. More specifically, among adolescents with elevated baseline depression, decreases in depression was an explanatory mechanism by which CBT led to improvements in 1-year BMI, adiposity, and insulin sensitivity. These significant conditional indirect effects, even in the absence of moderated mediation, provide valuable information about how intervention effects may differ depending on baseline depression, and are also consistent with previous findings indicating greater effects of depression interventions when baseline depression is elevated (Hayes, 2015; Stice et al., 2009).
Taken together, findings fit within a small, but growing body of literature investigating psychosocial interventions for depression, obesity, and T2D. Multidisciplinary interventions that target psychosocial mechanisms in addition to diet and physical activity may offer promise for reducing depression and BMI in adolescents with severe obesity (Jelalian et al., 2019; Nobles, Radley, Dimitri, & Sharman, 2016). Psychosocial interventions to address depression and glycemic outcomes in adults with diabetes have demonstrated a mix of positive and negative results (Friis, Johnson, Cutfield, & Consedine, 2016; Tovote et al., 2014; Uchendu & Blake, 2017). Given the major clinical challenges of intervening with young people with T2D (Nadeau et al., 2016), findings from the current study may have implications for how psychosocial interventions may play a role in preventing adverse health outcomes for adolescents in an applied healthcare setting. More specifically, findings suggest that preventive interventions targeting depression, especially CBT, for adolescents also presenting with elevated depression, might offer potential for ameliorating fasting insulin, a proximal risk factor for T2D, and lessening other adverse cardiometabolic outcomes (Singh et al., 2010).
Of note, it remains to be determined how decreases in depression affect BMI, adiposity, and insulin outcomes. Decreases in depression may alter emotional or disordered eating (e.g., binge eating), physical activity, and/or sedentary time, which could contribute to reductions in BMI and subsequent metabolic improvements (Moulton, Pickup, & Ismail, 2015). For instance, the association between depression symptoms and increased BMI over a 5-year period was mediated by emotional eating in adults (van Strien, Konttinen, Homberg, Engels, & Winkens, 2016). Decreasing depression symptoms also may contribute more directly to improved insulin outcomes via other pathways, such as cortisol-mediated physiologic stress response, inflammation, and/or improvements in sleep behavior and physiology (Moulton et al., 2015). For instance, reductions in depression symptoms over a 1-year period were associated with reductions in inflammatory biomarkers, such as high-sensitivity C-reactive protein, in adults with T2D (Herder et al., 2018). This represents a very important future direction for research examining risk and prevention of obesity and T2D.
One strength of the current study is the temporal independence of variables to examine mediation within the context of a randomized trial. With the exception of adiposity, examined changes in depression symptoms from baseline to post-treatment, and subsequent changes in BMI and insulin metrics from post-treatment to 1 year, minimizing the confound of data that overlap in time in the interpretation of effects (MacKinnon, Fairchild, & Fritz, 2007). In addition, this randomized control trial utilized a control condition matched for time, intensity, and leader competence. Consequently, observed indirect effects of intervention can likely be attributed uniquely to CBT, rather than expectancies or demand characteristics.
A shortcoming of this study is that the sample was not designed to have adequate power to test for simple mediation or moderated mediation. Post hoc power analyses are directly related to p values, and therefore should be interpreted with caution (Hoenig & Heisey, 2001). Yet, post hoc power analyses suggested 0.02–0.29 observed power for simple mediation and 0.07–0.54 observed power for indirect effects among adolescents with more elevated baseline depression. Thus, a sample of at least N = 120, all of whom had more elevated depression as opposed to more heterogeneous symptomatology, would be required to detect statistically significant indirect effects. An inadequately powered sample may have prevented the detection of statistically significant effects. Moreover, an inadequately powered sample may explain why we found significant conditional indirect effects, but not significant moderated mediation, for BMI, adiposity, and insulin sensitivity. Taken together, these findings merit testing in a larger sample with adequate power to determine whether baseline depression moderates the indirect effect of CBT on later outcomes through decreased depression. In addition, the effect sizes from the current study are small in magnitude, which may potentially limit the clinical significance of findings. That being said, the presence of small but significant effects can still communicate useful information regarding mechanisms of change, and therefore we believe the findings make a meaningful contribution to the literature (Agler & De Boeck, 2017). In addition, multiple statistical tests may increase the likelihood of type I error. Taken together, these secondary analyses require replication in a larger sample with greater power to detect direct and indirect effects.
Another potential shortcoming is that conclusions may only generalize to a subset of adolescent females at high risk for developing T2D. The specific and targeted approach of the current efficacy study is consistent with a precision medicine approach to address the heterogeneous mechanisms that explain risk for obesity and T2D (Kelly et al., 2018). Nonetheless, CBT has been found to be efficacious for both female and male adolescents in decreasing depression (Stice et al., 2008) and BMI (Jelalian et al., 2019). Future research is necessary to determine how psychosocial interventions might improve insulin outcomes for males at risk for T2D.
In summary, the current study makes an important contribution to the pediatric psychology literature by elucidating the role of a proposed causal mechanism–depression symptoms–on fasting insulin in a subgroup of high-risk adolescents. Applying moderated mediation to understand underlying mechanisms that explain an intervention’s effects represents a crucial step toward refining theoretical models of developmental pathology and increasing the success of specific and tailored interventions for reducing T2D risk (Kelly et al., 2018). CBT had significant indirect effects on fasting insulin 1-year following the intervention for those with elevated baseline depression symptoms via reductions in depression symptoms during the intervention phase. Future directions of this line of research might examine the mechanisms that explain the link of changes in depression with BMI/adiposity and insulin outcomes, including eating patterns, physical activity, sleep, and cortisol.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (Grant # K99HD069516 and R00HD069516); the National Institute of Health (NIH) Intramural Research Program Grant from NICHD (Grant # 1ZIAHD000641) to JAY, with supplemental funding from the NIH Bench to Bedside Program to LBS, MT-K, and JAY; Office of Behavioral & Social Sciences Research to JAY; and NIH Office of Disease Prevention to JAY.
Author Contributions
LDG and LBS wrote the manuscript. LDG led the conduct of data analysis. CHO and LBS assisted in data analysis and interpretation. LBS, MT-K, and JAY designed and obtained funding for the study. LBS and NK facilitated interventions and conducted assessments. ES trained interventionists. KYC oversaw body composition assessments. MT-K, JAY, NK, KYC, ES, CHO edited and commented on the manuscript and contributed to interpretation of findings.
Disclaimer
The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the PHS, USUHS, or the United States Department of Defense.
Declaration of interest: J. A. Yanovski is a Commissioned Officer in the United States Public Health Service (PHS). J.A. Yanovski reports his institution (NIH) has received grants to support clinical trials for which he is Principal Investigator unrelated to this research from Rhythm Pharmaceuticals, Zafgen Inc., and Soleno Therapeutics. No other authors have any financial or personal relationships to declare.
References
- Agler R., De Boeck P. (2017). On the interpretation and use of mediation: Multiple perspectives on mediation analysis. Frontiers in Psychology, 8, 1984. doi:10.3389/fpsyg.2017.01984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravender T. (2005). Health, education, and youth in Durham: HEY-Durham curricular guide (2nd edn.). Durham, NC: Duke University [Google Scholar]
- Friis A. M., Johnson M. H., Cutfield R. G., Consedine N. S. (2016). Kindness matters: A randomized controlled trial of a mindful self-compassion intervention improves depression, distress, and HbA(1c) among patients with diabetes. Diabetes Care, 39, 1963–1971. doi:10.2337/dc16-0416 [DOI] [PubMed] [Google Scholar]
- Gonzalez J. S., Tanenbaum M. L., Commissariat P. V. (2016). Psychosocial factors in medication adherence and diabetes self-management: Implications for research and practice. American Psychologist, 71, 539–551. doi:10.1037/a0040388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon T. S., Rofey D. L., Lee S., Arslanian S. A. (2013). Depressive symptoms and metabolic markers of risk for type 2 diabetes in obese adolescents. Pediatric Diabetes, 14, 497–503. doi:10.1111/pedi.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. F. (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76, 408–420. doi:10.1080/03637750903310360 [Google Scholar]
- Hayes A. F. (2015). An index and test of linear moderated mediation. Multivariate Behavioral Research, 50, 1–22. doi:10.1080/00273171.2014.962683 [DOI] [PubMed] [Google Scholar]
- Hayes A. F. (2018). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach (2nd edn). New York: Guilford Press. [Google Scholar]
- Herder C., Schmitt A., Budden F., Reimer A., Kulzer B., Roden M., Hermanns N. (2018). Longitudinal associations between biomarkers of inflammation and changes in depressive symptoms in patients with type 1 and type 2 diabetes. Psychoneuroendocrinology, 91, 216–225. doi: 10.1016/j.psyneuen.2018.02.032 [DOI] [PubMed] [Google Scholar]
- Hoenig J. M., Heisey D. M. (2001). The abuse of power: The pervasive fallacy of power calculations for data analysis. American Statistician, 55, 19–24. doi:10.1198/000313001300339897 [Google Scholar]
- Jelalian E., Jandasek B., Wolff J. C., Seaboyer L. M., Jones R. N., Spirito A. (2019). Cognitive-behavioral therapy plus healthy lifestyle enhancement for depressed, overweight/obese adolescents: Results of a pilot trial. Journal of Clinical Child & Adolescent Psychology, 48, S24–S33. doi:10.1080/15374416.2016.1163705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. S., Marcus M. D., Yanovski J. A., Yanovski S. Z., Osganian S. K. (2018). Working toward precision medicine approaches to treat severe obesity in adolescents: Report of an NIH workshop. International Journal of Obesity (London), 42, 1834–1844. doi:10.1038/s41366-018-0231-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D. P., Fairchild A. J., Fritz M. S. (2007). Mediation analysis. Annual Review of Psychology, 58, 593–614. doi:10.1146/annurev.psych.58.110405.085542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S., Friedman M. A., Arent S. M. (2008). Understanding the relation between obesity and depression: Causal mechanisms and implications for treatment. Clinical Psychologist, 15, 1–20. doi:10.1111/j.1468-2850.2008.00106.x [Google Scholar]
- Marshall W. A., Tanner J. M. (1969). Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood, 44, 291. doi:10.1136/adc.44.235.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton C. D., Pickup J. C., Ismail K. (2015). The link between depression and diabetes: The search for shared mechanisms. The Lancet Diabetes & Endocrinology, 3, 461–471. doi:10.1016/s2213-8587(15)00134-5 [DOI] [PubMed] [Google Scholar]
- Nadeau K. J., Anderson B. J., Berg E. G., Chiang J. L., Chou H., Copeland K. C., Zeitler P. (2016). Youth-onset type 2 diabetes consensus report: Current status, challenges, and priorities. Diabetes Care, 39, 1635–1642. doi:10.2337/dc16-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemiary D., Shim R., Mattox G., Holden K. (2012). The relationship between obesity and depression among adolescents. Psychiatric Annnals, 42, 305–308. doi:10.3928/00485713-20120806-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles J., Radley D., Dimitri P., Sharman K. (2016). Psychosocial interventions in the treatment of severe adolescent obesity: The SHINE program. Journal of Adolescent Health, 59, 523–529. doi:10.1016/j.jadohealth.2016.06.014 [DOI] [PubMed] [Google Scholar]
- O'Rourke H. P., MacKinnon D. P. (2018). Reasons for testing mediation in the absence of an intervention effect: A research imperative in prevention and intervention research. Journal of Studies on Alcohol and Drugs, 79, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden C. L., Kuczmarski R. J., Flegal K. M., Mei Z., Guo S., Wei R., Johnson C. L. (2002). Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics, 109, 45–60. doi:10.1542/peds.109.1.45 [DOI] [PubMed] [Google Scholar]
- Phillips G. A., Shadish W. R., Murray D. M., Kubik M., Lytle L. A., Birnbaum A. S. (2006). The Center for Epidemiologic Studies Depression Scale with a young adolescent population: A confirmatory factor analysis. Multivariate Behavioral Research, 41, 147–163. doi:10.1207/s15327906mbr4102_3 [DOI] [PubMed] [Google Scholar]
- Preacher K. J., Hayes A. F. (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods Instruments & Computers, 36, 717–731. doi:10.3758/Bf03206553 [DOI] [PubMed] [Google Scholar]
- Pulgaron E. R., Delamater A. M. (2014). Obesity and type 2 diabetes in children: Epidemiology and treatment. Current Diabetes Reports, 14, 508. doi:10.1007/s11892-014-0508-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurements, 1, 385–401. doi:10.1177/014662167700100306 [Google Scholar]
- Shaw M., Savoye M., Cali A., Dziura J., Tamborlane W. V., Caprio S. (2009). Effect of a successful intensive lifestyle program on insulin sensitivity and glucose tolerance in obese youth. Diabetes Care, 32, 45–47. doi:10.2337/dc08-0808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker L. B., Kelly N. R., Pickworth C. K., Cassidy O. L., Radin R. M., Shank L. M., Yanovski J. A. (2016). A randomized controlled trial to prevent depression and ameliorate insulin resistance in adolescent girls at risk for type 2 diabetes. Annals of Behavior Medicine, 50, 762–774. doi:10.1007/s12160-016-9801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker L. B., Kelly N. R., Radin R. M., Cassidy O. L., Shank L. M., Brady S. M., Yanovski J. A. (2017). Prevention of insulin resistance in adolescents at risk for type 2 diabetes with depressive symptoms: 1-year follow-up of a randomized trial. Depression and Anxiety, 34, 866–876. doi:10.1002/da.22617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Dhingra S., Ramdath D. D., Vasdev S., Gill V., Singal P. K. (2010). Risk factors preceding type 2 diabetes and cardiomyopathy. Journal of Cardiovascular Translational Research, 3, 580–596. doi:10.1007/s12265-010-9197-3 [DOI] [PubMed] [Google Scholar]
- Stice E., Rohde P., Gau J. M., Wade E. (2010). Efficacy trial of a brief cognitive-behavioral depression prevention program for high-risk adolescents: Effects at 1-and 2-year follow-up. Journal of Consulting and Clinical Psychology, 78, 856–867. doi:10.1037/a0020544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Rohde P., Seeley J. R., Gau J. M. (2008). Brief cognitive-behavioral depression prevention program for high-risk adolescents outperforms two alternative interventions: A randomized efficacy trial. Journal of Consulting and Clinical Psychology, 76, 595–606. doi:10.1037/a0012645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Shaw H., Bohon C., Marti C. N., Rohde P. (2009). A meta-analytic review of depression prevention programs for children and adolescents: Factors that predict magnitude of intervention effects. Journal of Consulting and Clinical Psychology, 77, 486–503. doi:10.1037/a0015168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote K. A., Fleer J., Snippe E., Peeters A. C. T. M., Emmelkamp P. M. G., Sanderman R., Schroevers M. J. (2014). Individual mindfulness-based cognitive therapy and cognitive behavior therapy for treating depressive symptoms in patients with diabetes: Results of a randomized controlled trial. Diabetes Care, 37, 2427–2434. doi:10.2337/dc13-2918/-/DC1 [DOI] [PubMed] [Google Scholar]
- Uchendu C., Blake H. (2017). Effectiveness of cognitive-behavioural therapy on glycaemic control and psychological outcomes in adults with diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Diabetes Medicine, 34, 328–339. doi:10.1111/dme.13195 [DOI] [PubMed] [Google Scholar]
- van Strien T., Konttinen H., Homberg J. R., Engels R. C., Winkens L. H. (2016). Emotional eating as a mediator between depression and weight gain. Appetite, 100, 216–224. doi:10.1016/j.appet.2016.02.034 [DOI] [PubMed] [Google Scholar]