Abstract
Caenorhabditis elegans has been an invaluable model organism in research fields such as developmental biology and neurobiology. Neurotoxicity is one of the subfields greatly profiting from the C. elegans model within biomedical context, while the corresponding potential of the organism applied to environmental studies is relevant but has been largely underexplored. Within the biomedical scope, the implication of metals and organic chemicals with pesticide activity (hereinafter designated as pesticides) in the etiology of several neurodegenerative diseases has been extensively investigated using this nematode as a primary model organism. Additionally, as a well-known experimental model bearing high sensitivity to different contaminants and representing important functional levels in soil and aquatic ecosystems, C. elegans has high potential to be extensively integrated within Environmental Risk Assessment (ERA) routines. In spite of the recognition of some regulatory agencies, this actual step has yet to be made. The purpose of this review is to discuss the major advantages supporting the inclusion of C. elegans in lower tiers of ERA. Special emphasis was given to its sensitivity to metals and pesticides, which is similar to that of other model organisms commonly used in ERA (e.g. Daphnia magna and Eisenia sp.), and to the large array of endpoints that can be tested with the species, both concerning the aquatic and the soil compartments. The inclusion of C. elegans testing may hence represent a relevant advance in ERA of chemicals, providing ecologically relevant insights towards improvement of the regulatory capacity for establishing appropriate environmental protection benchmarks.
Keywords: Caenorhabditis elegans, environmental risk assessment, neurotoxicity, pesticides, metals
Introduction
The discharge of trace elements and organic contaminants to the environment resulting from different human activities has been of utmost concern. Soil and aquatic compartments can be severely affected, as a result of direct discharge or indirect contamination following e.g. runoff and/or leaching (Petrie et al. 2014; Morrissey et al. 2015). Although the presence of contaminants in different environmental compartments does not indicate per se deleterious effects on organisms, cause-effect relationships may indeed exist. Consequently, the risk to human health and the environment related to contaminants presence in these compartments should be effectively assessed based on worldwide agreed upon regulatory standards. In this context, in the European Union (EU), the European Commission (EC) Regulation No 1907/2006 concerns the Registration, Evaluation, Authorization and restriction of Chemicals (REACH) based in risk assessment frameworks (EC 2018). Based on this regulation, it is likely ensured that substances, mixtures of substances and articles containing substances placed on the EU market do not cause adverse effects on human health and on the environment. This regulation originally considers all types of substances (although targeted regulation may then extend further on specific requirements), including metals and ingredients used in the formulation of pesticides, which are the two groups of contaminants focused upon in this article. In general, trace elements are regulated by REACH, and by the EC regulation No 1881/2006 that controls maximum levels of Pb, Cd, Hg and inorganic Sn in food (European Commission 2006). On the other hand, there is specific regulation with requirements and conditions for approval of biocidal Plant Protection Products [EC regulation No 1107/2009 (EC 2009)]. These can be defined as products mainly used in agriculture to protect crops against pests, mostly pesticides, but also other products such as growth regulators and pheromones. According to the EC regulation No 1107/2009, plant protection products (hereinafter generally referred to as pesticides for readability purposes) shall have no unacceptable effects on the environment, including contamination of surface and ground waters, air and soil, and they should specifically have no adverse impact on non-target species directly or indirectly exposed to the product.
To appropriately evaluate the risk that pesticides can pose to the environment, Environmental Risk Assessment (ERA) schemes are necessary, which are based in tiered approaches intending to assess the putative adverse effects and derive the regulatory acceptable concentrations. In general, similar approaches intending to protect human health and the environment from chemical threats are followed in the USA and Canada. In the USA, the Environmental Protection Agency (EPA) assumes the regulatory enforcement since the early 1970s under some federal laws. The Toxic Substances Control Act published in 1976 controls the production, importation, use and disposal of specific chemical substances and/or mixtures. Some chemicals such as pesticides are excluded from this act. Pesticides are rather regulated by the Federal Insecticide, Fungicide and Rodenticide Act. Also specifically dedicated, the Resource Recovery and Conservation Act lists and monitors a group of eight metals (As, Ba, Cd, Cr, Pb, Hg, Se and Ag. In Canada, pesticide protection goals are managed by the Health Canada’s Pest Management Regulatory Agency under the Pest Control Products Act and corresponding regulations (Pelaez et al. 2013; Canada 2018a; Canada 2018b; EPA 2018a; EPA 2018b; EPA 2018c; EPA 2018d).
As an example, the recommended framework for the ERA of pesticides in the EU, proposed by the European Food Safety Authority panel, is worthy of summarizing. For aquatic organisms in edge-of-field surface waters (Figure 1-A), four tiers (1–4) in ascending order of ecological realism and decreasing order of conservationism are used, all intending to achieve a given protection goal (EFSA 2013). Higher tiers are used when the lower tiers indicate unacceptable risk to the tested organisms by calculated risk quotients. These quotients are determined as the ratio between assessed effects and exposure likelihood, being the latter estimated on the basis of modelling approaches. In tiers 1 and 2, standard laboratorial tests with single species are used to assess effects (e.g. growth and reproduction inhibition), while an increase in the number of single species tested applies to the second tier. Tiers 3 and 4 include less standardized tests and are typically costlier, both in time and budget, since they require population/community studies (e.g. using micro and mesocosm approaches) and field studies, respectively. To evaluate the risk for in-soil organisms exposed to pesticides, the tiered approach is somewhat different (Figure 1-B). If the laboratorial studies performed in the lower tier indicate unacceptable risk, then field studies with realistic exposures are readily applicable in the highest tier (EFSA 2017). In the USA and Canada, these tiered approaches with increasing levels of refinement are also used, however the number and identification of the tiers is slightly different (tiers 1 to 3 and tiers 0 to 3 for USA and Canada, respectively) (Canada 2018a; Canada 2018b; EPA 2018a; EPA 2018b).
Figure 1.
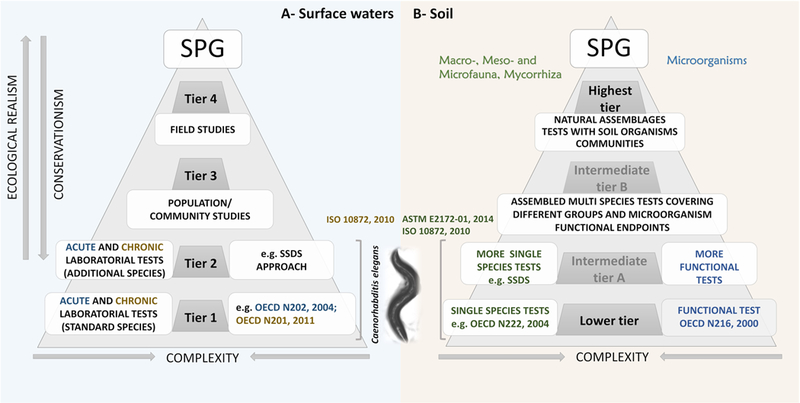
Tiered approach used in the environmental risk assessment of PPP for aquatic organisms in edge-of-field surface waters (A) and for in-soil organisms (B). All the tiers intend to achieve a defined specific protection goal (SPG). The nematode species Caenorhabditis elegans could be included in tiers 1 and 2 of the risk assessment regarding aquatic organisms (A) and in the lower tier and intermediate tier A of the risk assessment regarding soil organisms (B), using standard guidelines: ISO 10872 (ISO, 2010) (A) or ASTM E2172–01 (ASTM, 2014) and ISO 10872 (ISO, 2010) (B). In soil (part B), the toxicity assessment includes both tests with fauna and mycorrhiza and a microorganism-mediated process test. OECD N202 is the available guideline to perform the acute immobilization test with Daphnia Sp. (OECD, 2004a); OECD N201 to perform the growth inhibition test with freshwater alga and cyanobacteria (OECD, 2011a) and OECD N226 from 2004 to perform the reproduction test in soil with the mite Hypoaspis (Geolaelaps) aculeifer (OECD, 2004b). SSDs is the acronym for Species Sensitivity Distributions. The information used to build this figure was retrieved from EFSA reports (EFSA, 2013; EFSA, 2017).
Depending on the specific assessment context, as well as on the type of pesticide, different model organisms are recommended. For example, to evaluate the impacts of pesticides with an herbicidal mode of action at edge-of-field surface waters, the EFSA panel recommends the use of specific aquatic and sediment-dwelling organisms. The crustacean Daphnia sp. (OECD 2004a) and the fish Oncorhynchus mykiss (e.g. OECD 1992) are recommended when the focus is on acute toxicity. To assess chronic toxicity EFSA propose the use of a green algae such as Raphidocelis subcapitata (OECD 2011a), an additional non-green alga such as the diatom Navicula pelliculosa (OECD 2011a), a macrophyte such as Lemna sp. (OECD 2006), again Daphnia sp. (OECD 2008) and Chironomus riparius larvae (OECD 2011b) or the oligochaete Lumbriculus sp. (OECD 2007a) (EFSA 2013). Currently, to evaluate the impact of pesticides for in-soil organisms (EFSA 2017) the lower tier mostly considers a selection of invertebrate model species such as the earthworms Eisenia fetida or Eisenia andrei (ISO 2008; OECD 2015), the collembolans Folsomia candida or Folsomia fimetaria (ISO 1999; OECD 2009), the mite Hypoaspis aculeifer (OECD 2004b) and one microorganism-mediated process (N transformation) test (OECD 2000).
Specifically, regarding soil-directed risk assessment, it has been recognized that the standard organisms that have been recommended for testing might not be the most sensitive species, while groups such as the mycorrhiza, nematodes, terrestrial gastropods, enchytraeids and macrodetritivores are poorly represented (e.g. Bongers & Ferris 1999; De Vaufleury et al. 2006; EFSA 2017; Mallmann et al. 2018). In fact, the addition of a test with mycorrhizal fungi was recently proposed (EFSA 2017). Additionally, the nematode species Caenorhabditis elegans has been recognized as a meaningful add-on to the ecotoxicological test battery in pesticides ERA at least in the EU (EFSA, 2017), considering the availability of standard test guidelines to assess both acute (ASTM International 2014) and chronic (ISO 2010) toxicity. In fact, C. elegans and other nematodes have been widely presented as suitable ecological indicators for monitoring water (Boyd & Williams 2003a; ISO 2010) and soil (Peredney & Williams 2000; Höss et al. 2009; ASTM International 2014) quality. This nematode species should be included in test batteries used for the evaluation of pesticide impacts both regarding the water and soil compartments (specifically in lower ERA tiers), as detailed in the guideline ISO 10872 (ISO 2010) standardizing chronic tests with C. elegans in aqueous medium, sediments and soil (Figure 1-A and B). This guideline is designed for assessing chronic effects on growth, fertility and reproduction of the nematode, while the guideline ASTM E2172–01 (2014) supports acute toxicity assessment in soil (Figure 1-B), using lethality as an endpoint. Still, C. elegans has yet to be routinely included in ERA test batteries. Although no standard guideline to assess behavioral ecotoxicology and/or neuro-ecotoxicology is currently available for C. elegans, this organism could also be used under this context, as demonstrated by previous studies. For example, computer tracking methods were used to feasibly assess behavioral changes in the nematode as a response to exposure to different contaminants (e.g. Boyd et al. 2000; Dhawan et al. 2000; Cole et al. 2004). Others studies went further when addressing alterations in the locomotive behavior by investigating associations with dysfunction of the nervous system (Negga et al. 2012; e.g. Fagundez et al. 2015). The European Food Safety Authority, the Organization for Economic Co-operation and Development and the US Toxic Substances Control Act already recognized that there is a need to develop standardized protocols using models alternative to vertebrate animals (; EFSA 2009; EPA 2019) to assess effects of thousands of chemicals present in industrial, agricultural and consumer products in a time- and cost-efficient manner (Fritsche et al. 2015; e.g. Fritsche 2017; Bal-Price et al. 2018). A well-studied organism like C. elegans is at the forefront as candidate model in this context, especially to replace rodent testing.
Since 1960s that C. elegans has been a relevant experimental model organism in fields such as developmental biology and neurobiology. Its remarkable course in this context made it a nobelized worm once several Nobel Prizes have been awarded to researchers for discoveries based on C. elegans: Sydney Brenner, Robert Horvitz, and John Sulston earned the 2002 Nobel Prize in Physiology or Medicine; Andrew Fire and Craig Mello were awarded in 2006 with the Nobel Prize in Physiology or Medicine (Fire 2007; Mello 2007). Several characteristics, such as its easy maintenance in the laboratory, presence of a neuronal system with neuronal signal pathways and molecular networks shared with mammals, and the availability of its complete genome sequence, explain the growing and wide use of this organism as a model (Corsi et al. 2015). The use of C. elegans for research in medical and related fields is conspicuous, while its use in environmental sciences remains seldom and its potential is far from being recognized. An detailed search regarding topics related to the biomedical and environmental sciences fields in the scientific literature confirmed this picture (see Table S1 for details), showing that there are still few ecotoxicological studies with C. elegans (around 60 documents) compared to those developed within the scope of the biomedical sciences (more than 300 documents). It is notable that the studies related to neurodegenerative diseases are clearly collecting the highest relative frequency.
This review was built on the understanding that C. elegans utility in the biomedical fields does not extend to disciplines more strictly linking to environmental sciences, although both human health and ecological risk assessment are critical and mandatory to regulate the environmental safety of chemicals such as trace elements and pesticides. The overarching aim here was to collect relevant information about the studies that have been performed with this nematode, concerning their general purpose and specificities such as the contaminants, test platforms, test duration and endpoints assessed. Through this approach, we intended to show the range of applications available for the nematode towards clarification of different environmental research questions and its value as an add-on to ERA test batteries. Our attention was centered on pesticides and metals as major groups of contaminants of environmental concern.
Pesticides are chemicals specifically designed to affect biological targets in order to increase agricultural yield and, consequently, ensure the survival of human populations worldwide. Metals have been widely surveyed in different environmental compartments and bear the potential to bioaccumulate and biomagnify through the food web. Specific and tight environmental regulation is used worldwide to control these two groups of contaminants. Consistently, the literature review pointed out to these two classes as being the most commonly used in studies with C. elegans. A comparison of sensitivity to metals and pesticides was then done between the nematode species and two model invertebrates frequently used in ERA, in order to clarify its relative sensitivity and the applicability as an add-on to the ecotoxicological toolboxes supporting regulatory efforts. For these comparison purposes, the crustacean species Daphnia magna and the earthworm Eisenia fetida were selected, given their representativeness in surface waters and soil, respectively (Figure 1). The scope and aims of this review are addressed following a structured line of reasoning. First, C. elegans is characterized as a model organism, while the features supporting its use in environmental sciences are presented, with special attention being given to the neurotoxicity studies that can represent an important plus to ERA test batteries. Then, the versatility of this organism is exposed, elucidating on the possibilities for testing at different levels of biological organization and using diverse test platforms. Finally, the suitability of including C. elegans in ERA is addressed by appraising its relative sensitivity to the selected two classes of contaminants.
Features promoting C. elegans as a model species
Caenorhabditis elegans is a nematode of worldwide distribution in the wild. It is a small sized organism, with adults and its newly hatched larvae being 1 and 0.25 millimeters long, respectively. C. elegans can be isolated from organic-rich garden soils, compost and rotting vegetable matter since these substrates contain bacteria and small eukaryotes that the nematode can use as a food resource (Corsi et al. 2015; Frézal & Félix 2015). The organism can also be recovered from snails (e.g. Helix aspersa), since associations of this nematode species with terrestrial gastropods and other small soil organisms are common (Caswell-Chen et al. 2005; Schulenburg & Félix 2017). More rarely, C. elegans can also be found living in aquatic ecosystems (Majdi and Traunspurger 2015; Rasch et al. 2016; Abu Khweek and Amer 2018). It was described in the waterfront of saprobic rivers in Germany, in freshwater habitats in Italy and in aquatic biofilms in France (Kiontke 2006; Rasch et al. 2016). In fact, this species is abundant in microbe-rich environments (Schulenburg & Félix 2017). In the laboratory, the organism is normally cultured in agar plates with an added lawn of the bacterium Escherichia coli as food. In soil habitats, C. elegans has an important role in nutrient cycling and, consequently, in maintaining agricultural production, its sustainability and environmental quality, similarly to other soil invertebrates (Traunspurger et al. 1997; Höss et al. 1999; Lavelle et al. 2006).
The nematode was first proposed by Brenner (1974) as a genetic model in developmental biology and neurobiology fields. Over time, it has been contributing to answer several scientific questions, such as those related to the understanding of different molecular mechanisms in mammals (Yuan et al. 1993; Hengartner & Horvitz 1994; Horvitz 1999; Corsi et al. 2015). Nowadays, this species has developed into an important model for biomedical research, mainly in the functional characterization of novel drug-target interactions and in the identification of genes associated with particular pathways of toxic response or modes of toxic action, which may relate to specific diseases, e.g. the Alzheimer disease (Kaletta & Hengartner 2006). For example, C. elegans has been assuming a relevant role in the understanding of disorders such as Alzheimer’s disease and Parkinson’s disease, Diabetes type 2 and depression (Kaletta & Hengartner 2006; Harrington et al. 2010; Lublin & Link 2014), while supporting the discovery of drugs to be used in the development of therapy approaches (Lublin & Link 2014; Habchi et al. 2016). On the other hand, the use of C. elegans as an experimental model for ecotoxicity testing and ERA purposes has been relatively recent (over the past two decades) (Sochová et al. 2006; Höss et al. 2008; Höss et al. 2011; e.g. Hagerbaumer et al. 2015), and its applicability has been by far less explored compared to the other research fields (see table S1).
Table 1 depicts a summary with the strengths and limitations of C. elegans as an experimental model organism in biomedical research, as well as an appraisal of the features that can concomitantly be valuable for promoting the worm as a model in ERA. The promotion of this nematode species as a laboratory model in medical fields stems from several internal and external attributes that have been making the research with this species particularly fruitful. The knowledge of its complete genome sequence bearing conserved gene sequences, and the peculiar sharing of signaling pathways and cellular machinery for DNA replication and repair between C. elegans and humans (Table 1) are worth noting in this context. These are key attributes for the investigation of the modes of toxic action and disease pathways, which are frequently similar between humans and this invertebrate model (Cole et al. 2004; Kaletta & Hengartner 2006; Leung et al. 2008; Destefani et al. 2017). The available mutant and transgenic C. elegans strains (Table 1) are also very useful to mechanistically clarify modes of action, oxidative stress pathways, DNA damage patterns and neurodegeneration (Corsi et al. 2015; Hunt 2016). As an organism with metabolically active digestive, reproductive, endocrine, sensory and neuromuscular systems (Table 1), C. elegans responds as a functional multicellular and multi-systems unit in the toxicity assays. Furthermore, the logistics involved in culturing and testing with C. elegans is relatively easy to handle, which mostly derives from the species biological and physiological characteristics (Table 1). The nematode has a life cycle that extends only for 3.5 days when incubated at 20 °C (Figure S1). The organisms of this species are mostly self-fertilizing hermaphrodites, meaning that only one animal is needed to populate an entire plate. Only 0.1–0.2 % of the population produced by self-fertilization are males as consequence of the rare meiotic non-disjunction of the X chromosome, with most of the tests being carried out with hermaphrodites even though tests with males can also be done depending on the research hypothesis. As integrated in ERA, tests with hermaphrodites would thus be the most straightforward option following, with the advantage of supporting comparison to previous data, but sex-specific effects can also be tested particularly at higher ERA tiers where population and community studies are typically considered for a better and more direct insight on ecological responses (see Figure 1). C. elegans can be cultured within a large interval of temperature, ranging from 12 °C to 25 °C, meaning that its growth rate can be controlled by manipulating the temperature within this range. In-vivo toxicity assays with the organism can be performed within a short time period and using simple equipment. Moreover, they are cheaper and faster than the tests available with rodent models (Hunt 2016). All mentioned features are also valuable in the environmental sciences field, specifically regarding integration in ERA approaches.
Table 1.
Strengths and limitations of C. elegans as an experimental model organism. [ERA] is a code added to identify those features that prominently apply also to Environmental Risk Assessment.
| Strengths | Limitations |
|---|---|
| • Rapid and productive life cycle (≈3 days generation time and ≈300 progeny per hermaphrodite worm – primary form)1,2 [ERA] | • Become sterile at temperatures above 25 °C1 |
| • Not all metazoan genes are found in its genome | |
| • Organism transparency allowing a good visualization of internal structures under a microscope1,2 [ERA] | |
| • No C. elegans cell culture lines are commercially available1 | |
| • Small body size (around 1 mm long) and relatively simple physiology and anatomy1,2 | |
| • Experimental manipulation of individual tissues can be difficult due to its small size 1 | |
| • Easy and relative inexpensive maintenance in the lab, including to feed experiments1 [ERA] | |
| • Absence of some molecular pathways of mammals2 | |
| • Non-hazardous to lab workers2 [ERA] | |
| • Absence of several mammalian organs such as eyes, lungs, heart, kidney and liver2 | |
| • Exists primarily as a self-fertilizing hermaphrodite1,3 | |
| • Simple synchronization of organisms by isolation of newly hatched larvae or by treatment of gravid adults with bleach, with posterior isolation of the eggs1 [ERA] | |
| • Absence of adaptive immunity2 | |
| • The relatively impermeable cuticle limits toxicants internalization via direct dermal absorption2 [ERA] | |
| • Sexual crosses can be easily performed3 | |
| • Complete genome sequence available1 [ERA] | |
| • Small, unwanted changes in culture conditions, namely in temperature, salt and nutrients concentrations can alter assay results2 [ERA] | |
| • Good conservation of genes (60–80 % homologue genes), signaling common pathways and cellular machinery for DNA replication and repair between C. elegans and humans1,2,4 | |
| • Bad culture practices can result in altered gene expression patterns and accumulation of “dauer” larvae and males2 [ERA] | |
| • Mutant and transgenic C. elegans strains are readily available for many genes2 [ERA] | |
| • Reliable and reproducible DNA mutations are easily inducible3 | |
| • A small-scale C. elegans larval growth assay can be conducted in less than a week2 [ERA] | |
| • Possibility of long-term cryopreservation1 | |
| • Presents neuronal, motor, digestive, reproductive and endocrine systems2 | |
| • Shows endocrine signaling and sensory/behavioral responses to stimuli2 [ERA] | |
| • It is a well understood model organism, namely regarding its genetics, physiology, morphology, neurology and cell signaling pathways1,2 [ERA] |
The successful application of C. elegans as an experimental model depends also on an accurate perception of its limitations so that the experimental design can be properly tuned to the tested hypothesis with no uncontrolled interferences leading to uncertainty or feasibility problems (Table 1). For example, above 25 °C, the organisms become sterile, while shorter exposures to higher temperatures increase the production of males (Corsi et al. 2015), which may not comply to the desired experimental conditions; in extreme cases, poorly controlled culture practices favor the development of “dauer” larva, which are stress resistant alternatives to the common third larval stage (L3) (Hunt 2016).
Extending on C. elegans potential for broad-scope environmental studies: lessons learnt from human neurotoxicology
Shared neurophysiological components, cell functions, genes and pathways involved in DNA repair, as well as the mechanisms that are activated when the DNA is not repaired (e.g. cell cycle arrest and apoptosis), have made C. elegans an invaluable model to decipher important aspects of neurodegeneration and/or neurotoxicity (for specific cases in which the neurodegeneration is caused by toxic substances). As detailed in the previous section, C. elegans has been frequently used in an human neurotoxicology context (Cole et al. 2004; Benedetto et al. 2009; Harrington et al. 2010; Farina et al. 2014; Bailey et al. 2018). In contrast, studies investigating the neurotoxicity of contaminants in C. elegans under the environmental assessment scope are recent and scarce. It would be particularly useful to clarify the neurotoxicity of environmental compounds that were specifically designed to target the nervous system of biological pests. For example, organophosphates and carbamates are two sub-groups of insecticides specifically designed to inhibit irreversibly or reversibly the enzyme acetylcholinesterase (AChE), respectively. AChE inhibition interrupts the hydrolysis of the neurotransmitter acetylcholine, leading to its accumulation at the nerve synapses or neuromuscular junctions (Fukuto 1990). These insecticides have been shown to elicit adverse effects in non-target organisms such as earthworms (e.g. lethality, behavior alteration), which ultimately can be translated into decreased soil fertility and plant performance considering the ecological role of these organisms in agroecosystems (Reinecke & Reinecke 2007; Pereira et al. 2010; Pelosi et al. 2014). Similarly, the neurotoxicity of some trace elements [e.g. Hg and manganese (Mn)] to wildlife should not be disregarded, considering their occurrence in the environmental compartments. For example, Hg emissions were estimated to increase (Streets et al. 2009) and therefore neurotoxic effects in aquatic and soil organisms deserve attention.
The homology of the mammalian nervous system and other organisms support the hypothesis that the neurotoxicity of several contaminants as already well demonstrated in mammals could be also recorded in other groups of animals. ERA is an appropriate context where this hypothesis can be tested. There are general guidelines designed for neurotoxicity risk assessment both in USA and EU, but they have human health as the main focus (EPA 1998; OECD 2007b). The testing of the neurotoxic potential of chemicals is not mandatory by legislation of these countries and, when performed, it is generally based on the effects to adult rodents. Therefore, in the EU, both EFSA and OECD agreed that there is a need of developing a more standardized in vitro battery of tests to address the effects of chemicals on the nervous system for the final assessment of human toxicity (Fritsche et al. 2015; Fritsche 2017; Bal-Price et al. 2018). In summary, C. elegans has potential to be used in neurotoxicity assessment, not only in a human health context but also in an environmental health context as already recognized in other studies (Anderson et al. 2004; Cole et al. 2004; Fagundez et al. 2015).
The nervous system of hermaphrodite C. elegans, which has a total of 302 neurons, is subdivided in the pharyngeal nervous system with 20 neurons and the non-pharyngeal nervous system with 282 neurons (Figure 2) (Leung et al. 2008; Hunt 2016). Most of these neurons have a simple structure, presenting only one or two neurites (or processes), and most of these neurites cannot be distinguished as axons or dentrites because they both receive and transmit signals (Corsi et al. 2015). The use of C. elegans transgenic strains expressing fluorescent proteins in specific neurons allows in vivo visualization of virtually any desired neuron (Leung et al. 2008). The neurotransmitters found in the nematode and their main functions are summarized in Table 2. All these neurotransmitters except octopamine, as well as the neuronal signal pathways and molecular networks, are common to vertebrates (Leung et al. 2008; Hunt 2016). This includes neurotransmission, neuronal development and neuronal death or survival processes. Information obtained with C. elegans in studies evaluating alterations in these biological mechanisms can be used to interpret and infer regarding vertebrate systems based on the similarity the two groups for these processes. This possibility is paramount nowadays given the worldwide recognition of ethical principles that should rule animal experimentation and ensure the welfare of vertebrates. Alternative and complementary testing with C. elegans fits the replacement principle of the 3R’s philosophy, largely recommended in human and ecological risk assessment (e.g. Madden et al. 2013; Sneddon et al. 2017).
Figure 2.
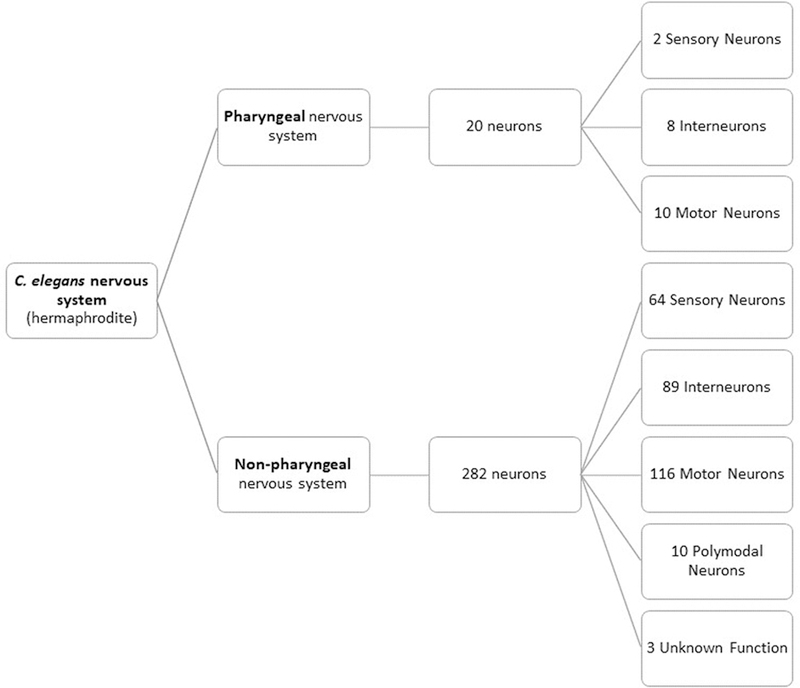
Division and classification of the neurons throughout the pharyngeal and non-pharyngeal nervous subsystems in C. elegans hermaphrodites. Adapted from McVey et al. 2012.
Table 2.
Identification and clarification of the major functions of the different neurotransmitters reported in C. elegans. Adapted from McVey et al. 2012.
| Neurotransmitters | Inhibition | Promotion |
|---|---|---|
| Octopamine | Egg laying Pharyngeal pumping |
— |
| Tyramine | Egg laying Pharyngeal pumping Spontaneous reversal |
— |
| Dopamine | Food-dependent forward movement | Slow movements in the presence of food High-angle turning Learning behaviors |
| Serotonin | Locomotion Defecation |
Pharyngeal pumping Egg laying |
| GABA | Muscle contraction | Muscle relaxation Defecation |
| Acetylcholine | — | Muscle contraction |
Validated testing platforms with C. elegans and endpoints at different levels of biological organization
A literature survey of the studies performed within neurotoxicology with C. elegans was done in order to provide an overview of the endpoints that have been assessed to address different research questions. These studies are based on different types of laboratorial assays assessing lethal and sub-lethal effects resulting from acute and chronic exposures to specific toxicants (e.g. Bailey et al. 2018). They regard mostly effects at the sub-cellular and cellular levels and, more rarely, the whole-organism. Examples of studies performed at different levels of biological organization are explored below.
Specifically considering sub-cellular effects (molecular endpoints), the neurotoxic potential of metals and pesticides in C. elegans has been investigated based on assays intending to measure the activity of acetylcholinesterase, oxidative stress or changes in gene expression. Cole et al. (2004) concluded that C. elegans can be a good model to the first-round screening of organophosphate-induced mammalian neurotoxicity, since cholinesterase inhibition occurred for 5 of 8 compounds tested, with an agreement between the ChE response of mammals and the worm in 7 out of 8 compounds. The occurrence of oxidative stress and mitochondrial inhibition have been signalled as relevant mechanisms of toxic action for some metals and associating to human neurological diseases (e.g. Parkinson’s disease), by evaluating mitochondrial function and/or quantification of reactive oxygen species (ROS) in C. elegans (e.g. Bailey et al. 2016; Todt et al. 2016): specific parameters such as oxygen and ATP concentrations and proton gradient integrity were used to assess the status of mitochondrial function after exposure; actual ROS quantification (e.g. superoxide, hydrogen peroxide, hydroxyl radical) and/or the monitoring of the up-regulation of genes encoding for enzymes with antioxidant activity, namely glutathione-S-transferase, superoxide dismutase, catalase and glutathione peroxidase were used to assess oxidative stress. Oxidative stress is widely studied in environmental assessment, being understood as both an early warning and a sensitive response of the soil and the aquatic biota to contamination challenges (e.g. Bernard et al. 2015; Brix et al. 2017; Wang et al. 2018; Qi et al. 2018). C. elegans can hence provide consistent information in this context as a nematode representative per se that can be compared/integrated with that provided by other ecological indicators. Furthermore, and importantly, the environmental assessment field can benefit from a well-developed testing platform allowing a detailed discrimination of common mechanisms of toxic action by environmental contaminants.
For example, to evaluate changes in the gene expression, modified strains of C. elegans are available, e.g. CL2166 strain (Bailey et al. 2016; Todt et al. 2016; e.g. Bailey et al. 2018). These strains are genetically modified such that a green fluorescent protein (gfp) gene is fused with a nuclear localization sequence driven by the promoter for the enzyme gene. These strains show increased fluorescence in the presence of oxidative stress that can be promoted by neurotoxicant substances (e.g. glyphosate, Mn). Microarray analyses (e.g. Custodia et al. 2001) and reverse transcription polymerase chain reaction (e.g. Zeng et al. 2017) are other techniques that have also been used for this purpose. Chronic exposure of C. elegans to concentrations of the fungicide Mn/Zn ethylene-bis-dithiocarbamate within the application range recommended by the manufacturer (thus relevant levels from the occupational exposure point of view), resulted in increased oxidative stress, namely mitochondrial Complex I inhibition with concomitant hydrogen peroxide production (Bailey et al. 2016). Similar results were obtained with glyphosate-containing pesticides as Bailey et al. (2018) verified that chronic exposure of C. elegans to a glyphosate-containing herbicide (TouchDown®, Syngenta) resulted in mitochondrial inhibition and hydrogen peroxide production. Fagundez et al. (2015) verified that acute exposure to Fe increased ROS levels in C. elegans, and Novillo et al. (2005) verified a change in the gene expression patterns as a consequence of a short-term exposure to Cd. Basically, this exposure activated the expression of genes encoding stress-responsive proteins, such as mtl-2, member of the metallothionein family, and cdr- 1, inducible for lysosomal protein (Novillo et al. 2005). Mechanistic approaches such as those exemplified above are highly valuable in environmental assessment to disentangle adverse outcome pathways of contaminants in the biota that may explain or predict escalating ecological effects. In this sense, and again, the toolbox available for C. elegans testing can be a valuable alternative or complement to current omics-based approaches currently used for the purposes with standard model organisms (e.g. Kim et al. 2015; Simões et al. 2019).
The number of studies focused on physiological and morphological endpoints in C. elegans is small, but results have provided important insights on the consequences of neurotoxic compounds at the morpho-functional level. For example, they helped in the evaluation of neurodegenerative effects, mostly related with exposure to pesticides (Meyer & Williams 2014; Todt et al. 2016; e.g. Bailey et al. 2018) and metals (e.g. Benedetto et al. 2009; Chen et al. 2013; Farina et al., 2014). Negga et al. (2011) verified that acute and chronic exposures of C. elegans to a glyphosate-containing herbicide and a Mn/Zn-ethylene-bis-dithiocarbamate fungicide lead to the formation of gaps between the nervous system and the inner cuticle, as well as to a thinning of the nerve cord. More recently, the authors confirmed that the exposure of the nematode to the same pesticides resulted in neurodegeneration of GABAergic and DAergic neurons (Negga et al. 2012). Stanwood et al. (2009) verified that chronic exposures to Mn can result in neurodegeneration of GABAergic and DAergic neurons within the basal ganglia, as well as Fagundez et al. (2015) observed that acute exposures to Fe resulted in morphological changes in dopaminergic and cholinergic systems.
Behavioral assays can detect alterations in the neuronal function that can be compromised by molecular and cellular alterations. Zeng et al. (2017) showed that the synthetic pyrethroid deltamethrin affects the locomotion (trashing rate, body bend frequency), foraging ability and egg-laying of C. elegans. The exposure to sub-lethal concentrations of the organophosphorus insecticide monocrotophos provoked paralysis (loss of wriggling movements) as well as decrease of the brood size of the worms (Leelaja & Rajini 2013). Moyson et al. (2018) evaluated the single and mixture effects of different metals, namely Cu, Cd and Zn on behavior (locomotion – average crawling speed and trashing behavior; chemosensation – chemotactic index, drop test) and lethality of the nematode. The authors observed that Cu was the most toxic metal, with higher mortality rates and reduced locomotion, and that some mixtures (Zn-Cu, Cu-Cd, and Zn-Cu-Cd) can increase the toxicity compared to what was observed in single exposures, decreasing the locomotion and increasing the lethality of the nematode. Fagundez et al. (2015) have shown that an acute exposure to Fe induced alterations in the survival, lifespan and brood size in C. elegans.
Overall, C. elegans has been widely used for testing the neurotoxicity of organic compounds and trace elements with the final aim of addressing human health effects These research efforts have been focused on molecular, morphological, physiological, behavioral and lethal endpoints, as summarized in Figure 3. Given the potential of C. elegans as highlighted previously, we specifically argue on the establishment of the nematode as a model organism for neuro-ecotoxicology assessment within lower tiers of ERA (Figure 1), comprising laboratorial tests (and endpoints) as illustrated in Figure 3. Additionally, C. elegans can be a good model also for studies involving higher levels of biological organization, namely in population and community studies (e.g. Haegerbaeumer et al. 2016) as required in higher ERA tiers (Figure 1), but further research is needed to confirm its suitability in this context.
Figure 3.
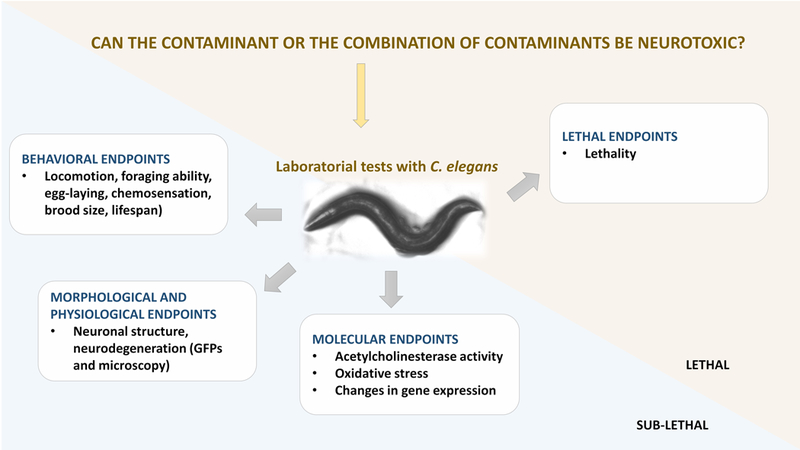
Summary of the sub-lethal and lethal endpoints that have been assessed in the laboratorial studies performed with the model organism C. elegans in an attempt to assess the neurotoxic potential of specific substances/mixture of substances. Endpoints are displayed by an increasing order of biological complexity.
C. elegans as a suitable test model in environmental assessment studies
As an inherent consequence of the genetic, physiological, and toxicological studies with C. elegans (see above), many and diverse molecular and biomedical tools are available and can be exploited for environmental assessment purposes (Menzel et al. 2009). Table 1 identifies the features of C. elegans as an experimental model that can also apply to ERA. This organism represents an advantage compared to other organisms already used in ERA given its suitability to be used in the assessment of neurotoxic substances (e.g. Cole et al. 2004; Negga et al. 2012); as well as serves as a model for both aquatic and soil ecosystems, mainly associated to microbe-enriched environments and occupying an intermediary position between microorganisms and higher trophic levels (Hagerbaumer et al. 2015). Microbe communities can act (1) as food themselves or as food supplier, processing substrate material to make it accessible to the nematode; (2) as part of its microbiome; (3) as parasites and pathogens; and (4) possibly as competitors. The organism can use macroscopic invertebrates such as slugs, snails and isopods as hosts or vectors to disperse into new habitats. Other nematode species and also small invertebrates can compete with C. elegans in the wild, and mites, collembola and fungi are its main predators (Schulenburg & Félix 2017).
Although relatively scarce and scattered, there are ecotoxicological studies in the literature with this organism, further supporting its utility as a valuable environmental indicator (Cioci et al. 2000; Roh et al. 2006; Ma et al. 2009). These studies are summarized in Tables 3 and 4. The 48 studies presented in table 4 assessed the toxicity of different contaminants (e.g. metals, pesticides, microplastics) to C. elegans and the 5 studies summarized in table 4 evaluated the toxicity of contaminated environmental matrices (soils and sediment) to the organism. The suitability of C. elegans to be successfully maintained in agar, soil, sediments and aqueous media supports the variety of setups used in the tests (e.g. Mutwakil et al. 1997; Black & Williams 2001; Höss et al. 2008). The worm’s applicability to both acute and chronic studies, intending to assess both lethal but also sub-lethal endpoints at different levels of biological organization is also evident (Tables 3 and 4). Growth (eg. Höss et al. 2001), reproduction (eg. Boyd & Williams 2003a), gene expression (eg. Anbalagan et al. 2012), locomotor and feeding behavior (Jiang et al. 2016), endocrine disruption (eg. Custodia et al. 2001) and enzyme activity are among the sub-lethal endpoints more frequently used in ecotoxicological studies performed with C. elegans. Neurotoxicity has been occasionally evaluated in an environmental context through endpoints such as locomotive and feeding behavior and expression of specific genes (Tables 3 and 4). It is noteworthy that most, if not all these endpoints are relevant in the environmental assessment context. Lethality, growth and reproduction of standard organisms (see ‘Introduction’) are frequently included as testing endpoints in lower ERA tiers (e.g. EFSA 2013). Behavioural endpoints reflecting in contamination avoidance and feeding inhibition are commonly used as early warning cues of exposure to noxious contaminants (e.g. Castro et al. 2018; de Santo et al. 2019; Gainer et al. 2019; Vidal et al. 2019), often reasoning neurotoxicological interference (e.g. Pereira et al. 2010; Bicho et al. 2015; Castro et al. 2018). Enzyme activity and endocrine disruption markers are also common endpoints both regarding the soil (Ibrahim et al. 2019) and the aquatic biota (Windsor et al. 2018; Li et al. 2016). Whether the responses of C. elegans compare to those by other model species typically used in ERA will be appraised in the following section of this manuscript.
Table 3.
Summary of ecotoxicological studies performed with C. elegans since 2000 where the nematode was found responsive to imposed toxicant challenges. The contaminants tested are indicated by grouping into major classes; in addition, the medium used, endpoints assessed and test length are given for each study, along with notes or conclusions as found relevant within the specific focus of the present review.
| Contaminants | Medium | Endpoints | Length | Notes/conclusions | References |
|---|---|---|---|---|---|
| Metals | |||||
| Cd, Cu, Ni, Pb, Zn | Soil | Lethality | 24 h | The soil type used in the ecotoxicological tests with C. elegans can influence the results | (Boyd & Williams 2003b) |
| Cu-Zn, Cu-Cd, Cd-Pb (mixtures) |
Soil | Growth | 1-week | The simple additivity model underestimated mixture effects on reproduction and body length of C. elegans. | (Jonker, Sweijen, et al. 2004) |
| Zn, Cd, Hg, Cu, Fe, Cr, As, Al, Ni, Mn, Pb | Soil pore water | Gene expression | 40 h | C. elegans GFP-reporter strains can be very useful in lower tier ecotoxicological screening | (Anbalagan et al. 2012) |
| Cu | Soil Aqueous medium |
Lethality, reproduction, movement, feeding behavior | 24 h; 72 h; 24 h; 24 h | C. elegans exhibited intermediate sensitivity compared to two other nematode species | (Boyd & Williams 2003a) |
| Cu | Aqueous medium Soil |
Lethality, behavior | 24 h; 24 h | C. elegans carrying a GFP-expressing element responded similarly to the wild-type N2 strain | (Graves et al. 2005) |
| Cd | Aqueous medium Soil |
Growth | 72 h | -- | (Höss et al. 2001) |
| Cu, Cd, Zn, Pb, Ni | Soil | Lethality | 24 h | C. elegans and Eisenia fetida have a similar response to metals | (Peredney & Williams 2000) |
| Cu, Cd | Agar | Reproduction, larval growth, lifespan | 36 h; 32 h | -- | (Kurauchi et al. 2009) |
| Cd, Cu | Agar | Reproduction, larval growth, lifespan | 36 h; 32 h | -- | (Harada et al. 2007) |
| Cd-Cu | Agar | Reproduction, length of the juvenile period, length of the reproductive period, body length | ≈13 days | -- | (Jonker, Piskiewicz, et al. 2004) |
| Al, Cu, Pb, Zn, Cd | Aqueous medium | Lethality, behavior | 24 h; 24 h | A computer tracking system was successfully used to measure several behavioral parameters in C. elegans | (Dhawan et al. 2000) |
| Cu, Zn, Cd, Cr | Aqueous medium | Growth, reproduction, behavior (head thrash frequency, body bend frequency, feeding), enzymatic quantification | 24 h; 72 h; 24 h; 24 h; 24 h | Considering simplicity, accuracy, repeatability and costs of the experiments, feeding inhibition is a good sub-lethal endpoint for assess metal toxicity using C. elegans, | (Jiang et al. 2016) |
| Cd, Hg, Cu, Pb, Cr, Ni, Al, Co, Zn, Mn (single and mixtures) | Aqueous medium | Lethality | 96 h | C. elegans KC136 strain allows rapid monitoring of environmental hazards | (Wah Chu & Chow 2002) |
| Pb, Cu, Cd | Aqueous medium | Movement, feeding, growth, reproduction | 4 h; 24 h; 72 h |
Differential sensitivity among endpoints for any metal after 4 h but not after 24 h | (Anderson et al. 2001) |
| Cu, Pb, Al | Aqueous medium | Locomotor behavior | 4 h | -- | (Anderson et al. 2004) |
| Cd | Aqueous medium | Gene expression | 4 or 7 days | -- | (Novillo et al. 2005) |
| Cu | Aqueous medium Soil |
Maturity index | 24 h | The maturity index responds to metal pollution and thus may serve as an indicator of soil health | (Bongers et al. 2001) |
| Zn | Sediment Aqueous medium |
Reproduction, lethality | 96 h, 48 h | -- | (Haegerbaeumer et al. 2016) |
| Zn | SedimentAqueous medium | Abundance, species richness | 6 months | Inhibitory effects of Zn on abundance and species richness occurred only in the higher concentration (100 mg Zn/Kg d.w. soil) | (Haegerbaeumer et al. 2016) |
| Organic compounds | |||||
| Atrazine, PCB52, fluoranthene, lansoprazole | Aqueous medium | Reproduction, gene expression | 96 h | -- | (Menzel et al. 2005) |
| Carbendazim | Agar | Reproduction, length of the juvenile period, length of the reproductive period, body length | ≈13 days | -- | (Jonker, Piskiewicz, et al. 2004) |
| Pentadecafluorooctanoic acid, dichlorvos, sodium dodecyl sulfate | Agar | Reproduction, larval growth, lifespan | 36 h; 32 h | -- | (Kurauchi et al. 2009) |
| Sodium dodecyl sulfate | Agar | Reproduction, larval growth, lifespan | 36 h; 32 h | -- | (Harada et al. 2007) |
| 4-Nonylphenol | Aqueous medium | Growth, reproduction | 72 h | Growth and reproduction of C. elegans were enhanced at concentrations of 66 and 40 μg/L of 4-Nonylphenol | (Höss et al. 2002) |
| Glyphosate, paraquat, 2,4-dichlorophenoxyacetic acid, cypermethrin, carbendazim dichlorvos, endosulfan, diuron, chlorpyrifos | Aqueous medium | Gene expression, feeding | 40 h; 28 h | Transgenic GFP-reporter strains may be useful to assess stress patterns regarding gene expression in response to pesticides | (Anbalagan et al. 2013) |
| Quinoline, acridine, phenazine, and 1,10-phenanthroline, toxaphene, hexachlorobenzene, short-chain chlorinated paraffins | Soil Agar Aqueous medium |
Mortality | 24 h; 48 h | Recommended inclusion of C. elegans in the battery of tests for ERA of persistent organic pollutants in soil | (Sochová et al. 2007) |
| Carbendazim, carbendazimiprodione | Soil | Growth | 1-week | -- | (Jonker, Sweijen, et al. 2004) |
| Progesterone, estradiol, cholesterol | Aqueous medium | Gene expression | 4 or 7 days | -- | (Novillo et al. 2005) |
| DMSO, acetone, ethanol, mebendazole, levamisole, chlorpyrifos | Aqueous medium | Locomotor behavior | 4 h | -- | (Anderson et al. 2004) |
| Cholesterol, estrogen, testosterone, progesterone | Aqueous medium | Vitellogenin expression | 3–4 days | C. elegans may be a useful laboratorial and field model for screening endocrine disruptors | (Custodia et al. 2001) |
| Cholesterol, 4- nonylphenol, p-octylphenol, triphenyltin chloride, tributyltin chloride | Agar | Reproduction, fecundity | 120 h | C. elegans fecundity and reproduction rates can be good endpoints to assess effects of alkylphenols and organotin compounds | (Tominaga et al. 2002) |
| 17β-Estradiol, Bisphenol, Tributyltin Chloride | Agar | Number of germ cells | 6 days | C. elegans can be a good model to determine the reproductive toxicity of contaminants | (Hoshi et al. 2003) |
| Atrazine, fluoranthene, diethylstilbestrol, β-naphthoflavone, clofibrate | Aqueous medium | Gene expression | 48 h | Gene expression of C. elegans induced by contaminants can be a good tool to detect responses at a transcriptional level | (Reichert & Menzel 2005) |
| Bisphenol A | Aqueous medium | Growth, locomotion behavior, lifespan, lipofuscin accumulation, stress-related genes expression, population size | 10 d | Chronic exposures of C. elegans to Bisphenol A caused adverse effects in all endpoints measured, including population size | (Zhou et al. 2016) |
| Bisphenol A | Aqueous medium | Growth, reproduction, locomotion behavior, stress-related genes expression | 96 h x4 | C. elegans was successfully used to conduct multigenerational testing | (Zhou et al. 2016) |
| Vinyl chloride | Aqueous medium | Survival, reproduction, stress-related gene expression | 72 h | Reproduction and gene expression were the most sensitive endpoints | (Nam & An 2010) |
| Chlorpyrifos | Aqueous medium | Lethality, stress-related gene expression, acetyl-cholinesterase activity, growth, reproduction, development | 24 h; 24 h; 24 h 24 h; 96 h | -- | (Roh & Choi 2008) |
| Insecticidal Cry3Bb1 protein | Soil Aqueous medium |
Growth, reproduction and gene expression | 96 h; 96 h; 16 h, 24 h and 48 h | -- | (Höss et al. 2011) |
| Mixtures of metals and pesticides | |||||
| Cu–carbendazim, Cu–carbendazimiprodione | Soil | Growth | 1-week | -- | (Jonker, Sweijen, et al. 2004) |
| Cu–carbendazim | Agar | Reproduction, length of the juvenile period, length of the reproductive period, body length | ≈13 days | -- | (Jonker, Piskiewicz, et al. 2004) |
| Nanoparticles | |||||
| ZnO-NPs | Aqueous medium | Growth, locomotive behavior, metabolic ATP level, oxidative stress | 72 h; 72 h; 66–67 h; 72 h | Sub-lethal metabolic and locomotive endpoints of C. elegans allow assessing ecotoxicity of nanoparticles in aquatic environments | (Huang et al. 2017) |
| Ag-NPs | Agar | Survival, growth, reproduction, stress-response gene expression | 24 h; 24 h; 72 h; 24 h | -- | (Roh et al. 2009) |
| ZnO-NPs, Al2O3-NPs, TiO2-NPs | Aqueous medium | Lethality, length, number of eggs, offspring per worm | 24 h; 5 d; 5 d; 5 d | -- | (Wang et al. 2009) |
| CeO2-NPs, TiO2-NPs | Agar | Stress- response gene expression, growth, fertility, survival | 24 h | -- | (Roh et al. 2010) |
| Ag-NPs | Aqueous medium | Growth | 24 h, 48 h, 72 h | -- | (Bone et al. 2015) |
| Sulfidized Ag-NPs | Aqueous medium | Lethality | 24 h | -- | (Collin et al. 2016) |
| Ag-NPs | Soil | Locomotive behavior; bioaccumulation | 65 h; 24h and 48h | C. elegans can be an effective organism to rapidly screen and assess the impacts of metal nanoparticles on soil environment | (Yang et al. 2017) |
| Microplastics | |||||
| Polyamides, polyethylene, polypropylene, polyvinyl chloride, polystyrene particles | Agar | Survival, body length, reproduction, intestinal damage, oxidative stress | 2 d | Microplastic particles induced effects in all endpoints measured in C. elegans | (Lei et al. 2018) |
Table 4.
Summary of ecotoxicological studies performed with C. elegans since 2000 in matrices collected from the field. Only studies where the nematode was responsive to the imposed toxicant challenges where included and the contaminants burdening the tested matrices are indicated. Basic test features (medium, endpoints, test length) are given, along with notes or conclusions as found relevant within the specific focus of the present review.
| Tested matrices | Contaminants | Test medium | Endpoints | Test length | Notes/conclusions | References |
|---|---|---|---|---|---|---|
| Contaminated freshwater sediments | Co, Ni, Cu, Zn, Cd, Hg, Pb | Aqueous extract of the sediments | Survival, growth, locomotion, gene expression | 24 h; 48 h; 24 h; 4 h; 24 h | C. elegans testing allowed to identify patterns of toxicity along Magdalena river | (Tejeda-Benitez et al. 2016) |
| Several metals and organic pollutants (e.g. Hg, Zn, As, Cr, tributyltin, petroleum hydrocarbons) | Freshwater sediments | Reproduction, gene expression, endocrine disruption | 96 h; 48 h | Gene expression in C. elegans should not replace conventional aquatic monitoring techniques, but can be a good complement | (Menzel et al. 2009) | |
| Contaminated soils | Several metals and organic pollutants (e.g. polycyclic aromatic hydrocarbon, Cd, Cr) |
Soil | Fertility, growth and reproduction | 96 h | C. elegans was more affected by organic than by metal contaminants | (Höss et al. 2009) |
| Cd, Cu, Zn, Pb | Soil | Lethality | 24 h | _____ | (Black & Williams 2001) | |
| Di(2-ethylhexyl)phthalate | Soil | Mortality, growth, reproduction, stress-related gene expression |
24 h | Growth, reproduction and stress-related gene expression were the most responsive endpoints | (Roh et al. 2007) | |
The objectives and scope of the ecotoxicological studies that have been carried out in C. elegans are quite diverse (Tables 3 and 4). For example, Black and Williams (2001) assessed the mortality of C. elegans and of the amphipod Hyalella azteca when exposed to soil and sediment contaminated after a release of trace elements from a mine waste lagoon to the Hungarian Tisza river (acute toxicity; whole-organism level). The authors reinforced the ease and utility of C. elegans in soil toxicity tests, which, on the one hand, involves the use of lower quantities of soil compared to other soil toxicity tests with other organisms and, on the other hand, allows to retrieve large sets of information. Tejeda-Benitez et al. (2016) evaluated the toxicity profiles of freshwater sediments contaminated by metals (Co, Ni, Cu, Zn, Cd, Hg and Pb) through endpoints such as survival, locomotion, growth (wild-type strain) and changes in gene expression (GFP transgenic strains) of C. elegans, thus addressing acute and chronic toxicity, as well as the sub-cellular and whole-organism levels. Tominaga et al. (2002; 2003) studied the effects of different substances and compounds (steroid hormones, alkylphenol and organotin compounds) in the reproduction of over multiple generations of C. elegans in agar plates (chronic toxicity; whole-organism level) and showed that the effects can persist over generations, highlighting the nematode as a good model for multigenerational studies and epigenetic effects.
The contaminants that have been tested in ecotoxicological studies with the nematode species are also diverse. For example, Höss et al. (2011) analyzed the environmental hazardous potential of the protein Cry3Bb1, which is produced by a genetically modified maize and presents insecticidal properties against the coleopteran Diabrotica virgifera, using C. elegans as a non-target representative. The authors observed that even though the Cry3Bb1 exerted dose-dependent inhibitory effects on growth and reproduction of the nematode, the concentrations found in crop soil were four orders of magnitude below the toxicity threshold, indicating low hazardous potential at least to C. elegans.
Transgenic strains of C. elegans can be used for mechanistic ecotoxicity assessment through integrating the response of different biomarkers. For example, Tejeda-Benitez et al. (2016) evaluated changes in the gene expression of C. elegans as a response to the exposure to freshwater sediments contaminated by metals, using several transgenic nematode strains (hsp-6::gfp, hsp-16.2:: gfp, hsp- 70:: gfp, sod-1:: gfp, sod-4:: gfp, gpx-6:: gfp, mtl-2:: gfp, mtl-1:: gfp, gst-1:: gfp; cyp-34A9:: gfp). They detected changes in the expression of the mtl-2 gene (metallothioneins-related gene), indicating contamination by metals, as already expected; antioxidant gene sod-4, indicating possible presence of compounds responsible for the generation of ROS; and metabolism-related genes sod-4 that have been frequently activated by the presence of pesticides and other xenobiotics. Chu and Chow (2002) evaluated the mortality caused by trace elements and compared the results between the wild-type nematode and a transgenic strain (KC136), carrying a heat shock promoter driven GFP reporter gene. They concluded that the transgenic strain revealed the same sensitivity to metals that the wild-type, meaning that the transgenic KC136 could be used to early and rapidly detect pollution by metals in situ (Wah Chu & Chow 2002; Tejeda-Benitez et al. 2016).
Although most of the tests with C. elegans have been performed using a single-species approach, these organisms are referred as suitable to integrate more holistic test approaches such as those based in microcosm and mesocosm experiments (Bone et al. 2015; Hagerbaumer et al. 2015). For example, Haegerbaeumer et al. (2016) used C. elegans in a microcosm study containing sediment, along with other nematode species, to investigate direct and indirect effects of Zn on natural nematode assemblages. They concluded that testing the toxicity of Zn under more realistic exposure conditions revealed a species sensitivity ranking comparable to field observations, confirming that assessments at these higher levels of biological organization represent a more sensitive approach to realistically address the effects of the chemicals. It also can render more realistic the assessment of environmental interference in the response to chemical challenges. For example, Collin et al. (2016) showed that natural organic matter alters the toxicity of sulfurized Ag nanoparticles to C. elegans since its dissolution is influenced by the organic matter. The use of the nematode species in microcosm and mesocosm studies would also be an important asset for assessment at higher tiers of ERA (EFSA 2013).
Comparative sensitivity between C. elegans and standard ERA models
C. elegans has been shown to be highly sensitive to different environmental contaminants, namely to some metals and pesticides (see Table S2 for a summary list denoting sensitivity). Despite this has been generally argued, studies directly comparing its sensitivity with standard laboratorial test organisms are still scarce. While the ecological representativeness of the organism, knowledge on its physiology and the availability of suitable testing platforms are key features of a valuable candidate to integrate ecotoxicological test batteries for ERA purposes, its sensitivity is of no lesser importance given the relevance of the precautionary principle in environmental assessment (e.g. EFSA 2013). This motivated us to run a comparative analysis, confronting the responses of C. elegans to different contaminants with their counterpart recommended models in ERA focusing on soil (E. fetida) or aquatic (D. magna) ecosystems. The raw results of these analyses are available in supplementary tables S2–S4, while figure 4 provides a graphical comparative view of sensitivity to metals, as detailed later in this section.
Figure 4.
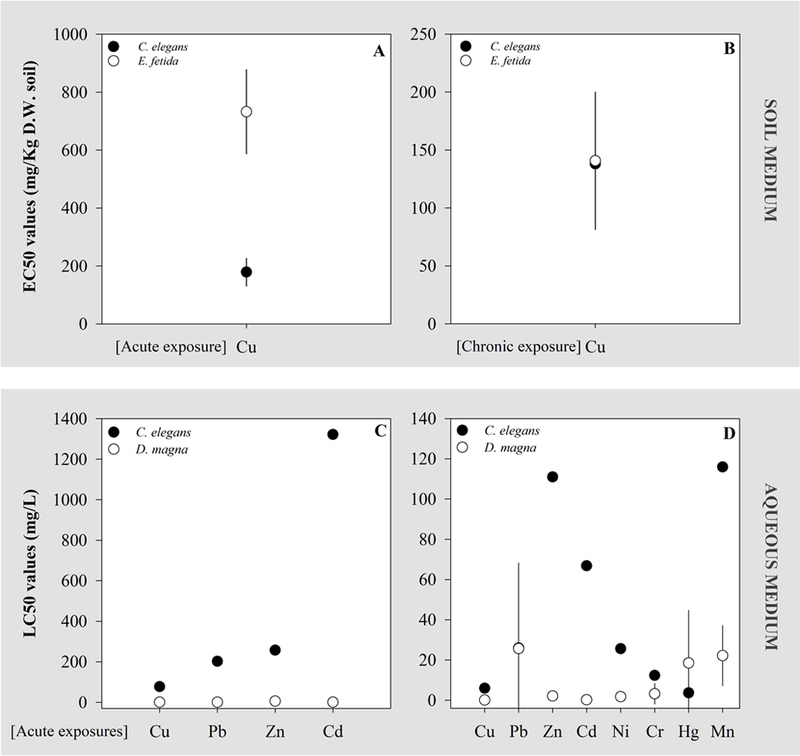
Comparative view on the sensitivity to several environmental contaminants of C. elegans and that by two standard organisms used in ecotoxicological test batteries within environmental risk assessment frameworks, Eisenia fetida and Daphnia magna, representing soil and aquatic ecosystems, respectively. At the top panel, comparison of (A) acute EC50 values (effect: lethality following 24 h exposure for C. elegans and 14 days for E. fetida), and (B) chronic EC50 values (effects: reproduction following 7 days for C. elegans and 28 days for E. fetida. These effective concentrations were selected from laboratorial studies performed in soil (see tables S2 and S4; Boyd et al., 2010; Jonker et al., 2004b; Spurgeon et al., 1994; U.S. Environmental Protection Agency ECOTOX database, 2018). At the lower panel, comparison of (C) 24 h-LC50 and (D) 48 h-LC50 values found for exposures of C. elegans and D. magna to metals. These LC50 values were selected from laboratorial studies performed in aqueous media (see tables S2 and S3; Altındag et al., 2008; Boyd and Williams, 2003a; Dhawan et al., 2000; Ferreira et al., 2008; Graves et al., 2005; Sorvari and Sillanpää, 1996; Wah Chu and Chow, 2002), and when several values were available for a given element, average between available values and corresponding standard deviation were used to obtain the plotted information.
Peredney et al. (2000) showed that C. elegans was more sensitive to trace elements contamination than other model soil organisms frequently used for ecotoxicological assessment purposes, namely E. fetida and F. candida. The authors compared effective concentrations obtained following a 24-h exposure of C. elegans with equivalent benchmarks obtained following tests lasting for up to 14 days with E. fetida. The dramatic shortening of test length with equivalent or more environmentally protective outcomes is an additional argument in favor of considering C. elegans testing within ecotoxicological batteries for risk assessment purposes. Höss et al. (2009) also assessed the toxicity of soils contaminated by trace elements and organic compounds to the nematode, and compared to the results obtained in tests with other test organisms (F. candida, Eisenia andrei and Brassica rapa). They reported that C. elegans was, in most cases, the most sensitive species to soils contaminated with PAH, compared to E. fetida and F. candida, based on the reproductive endpoint. Exceptions to this general pattern revealed equivalent sensitivity to F. candida, or sensitivity close to that of the two other organisms. Other organic contaminants such as quinoline, acridine, phenazine, 1,10-phenanthroline and toxaphene were found to be similarly or occasionally less lethally toxic to C. elegans than E. fetida and F. candida (Sochová et al. 2007). Daam et al. (2011) showed that nematodes in general were more sensitive to two fungicides containing Cu than E. fetida.
Considering the tests performed in soil medium since 2000, a sensitivity comparison between C. elegans and the earthworm E. fetida (tables S2 and S4) was found fruitful for Cu. This was the combination selected because E. fetida is a recommended model soil organism in ERA and, importantly, there is data available for both cases with comparable units, endpoints, test medium and test type. A graphical comparison between EC50 Cu values (effective concentrations of Cu causing 50% of effect on the tested species) obtained for both species shows that, considering the endpoint lethality, C. elegans was more acutely sensitive to Cu than the earthworm (Figure 4-A). This is in agreement with the conclusions of Daam et al. (2011). Based on reproductive effects following chronic exposures to Cu, the sensitivity was similar for the two organisms (Figure 4-B). Still, the use of the nematode instead of the earthworm in risk assessment approaches targeting Cu would be more advantageous both considering acute and chronic assays, in the latter case due to the relevant shortening of the test length. It is noteworthy that the use of only E. fetida as environmental indicator for the soil compartment regarding contamination by Cu may not be appropriate, since concentrations of Cu that are safe for E. fetida can compromise the short-term survival of C. elegans.
Figure 4 (C, D) shows a comparison between the sensitivity of C. elegans and D. magna regarding 24 h-LC50 and 48 h-LC50 values (lethal concentrations causing mortality of 50 % of the tested organisms, 24 h and 48 h after the beginning of the test, respectively) for different metals, namely Cu, Pb, Zn, Cd, Ni, Cr, Hg and Mn. Following 24 h of exposure, D. magna was generally more sensitive than C. elegans to all metals. The lower and higher differences in sensitivity occurred for Cu and Cd, respectively. On the other hand, the sensitivity to Cu, Pb and Cr was very similar for both organisms following 48 h of exposure, with C. elegans being apparently more sensitive than D. magna to Hg (Figure 4-D). In fact, D. magna was generally more sensitive to metals, when compared to C. elegans, suggesting that the inclusion of the nematode in test batteries may not be a valuable add-on to the aquatic risk assessment of these specific metals, especially in lower tiers where lethality can be inspected. However, the set of data available for comparison is very limited, preventing robust conclusions in this context. Moreover, C. elegans actually represents a different functional group in aquatic ecosystems, which may render its inclusion in test batteries valuable per se.
Ecotoxicological studies with pesticides for C. elegans, E. fetida and D. magna are very scarce (Tables S2–S4), severely limiting any attempt to conclude on the differential sensitivity of organisms; for chlorpyrifos, D. magna feeding at 24 h (average EC50 = 0.450 ±0.066 mg/L) was more sensitive that C. elegans locomotive behavior at 4 h (EC50 = 1.750 mg/L) (see tables S2 and S3; Anderson et al., 2004; Loureiro et al., 2010), although the endpoints where inherently different since filtration in Daphnia is a more passive process than C. elegans locomotion (Mcmahon 1965; e.g. Corsi et al. 2015). Still, C. elegans has been proven to be very sensitive to some pesticides (e.g. carbamates, organophosphates) in neurotoxicological studies within the human toxicology field.
Conclusions and future perspectives
The contribution of C. elegans to scientific advances in the medical science field is unequivocal, namely by supporting many important findings ranging from genetics, molecular biology to physiology and neurotoxicology. Although C. elegans can be very useful as a model to other fields of research, namely those related with environmental sciences, its applicability has yet to materialize. Nevertheless, several studies have highlighted the potential utility C. elegans might have in ecotoxicological studies with trace elements and pesticides. Notably, standardized protocols for toxicity tests with C. elegans in several environmental matrices are already in place. These observations provided the foundation for the current review, for supporting the integration of C. elegans into ecotoxicology test batteries within ERA frameworks. As described above, C. elegans may be used both in acute and chronic studies, which are requirements of the lower tiers of ERA, representing a more efficient model than standard soil organisms currently used in these procedures (e.g. E. fetida for soil ERA). Tests with C. elegans can have a shorter duration (both for acute and chronic exposures) than those performed with other organisms, while presenting a higher or equivalent sensitivity to several toxicants. Moreover, effects of environmental contaminants can be assessed in the nematode at different levels of biological complexity (from sub-cellular and cellular levels to whole-organism), commensurate with the utility of the nematode as an established model in neurotoxicology.
Finally, it is worth emphasizing that the nematodes group has yet to be integrated as a model organism represented in ERA ecotoxicological test batteries. This vision has been corroborated by at least one expert group reporting to a European regulatory agency, namely EFSA, which has considered C. elegans as a potentially relevant model organism to be used in this context (EFSA 2017). This review supports the assertion that C. elegans is a valuable model to be considered for ERA, particularly for lower tiers targeting the soil compartment. Studies investigating the overall potential of the organism in microcosm and mesocosm approaches are necessary to further support the utility of C. elegans as a model for higher tiers of ERA.
Supplementary Material
Acknowledgements.
The authors are grateful to two anonymous reviewers; their constructive comments supported the improvement of the manuscript.
The author’s affiliation is as shown on the cover page. The authors participated in the development of the paper as individual professionals and have sole responsibility for the writing and content of the paper. None of the authors have been involved in the last five years with regulatory or legal proceedings related to the contents of the paper. The study was funded by institutional and individual grants as follows. FCT/MCTES provided financial support through national funds to CESAM (UID/AMB/50017/2019), LQ (SFRH/BD/129871/2017), as well as JLP and PP (within the scope of the framework contract foreseen in article 23 of the Decree-Law 57/2016, changed by Law 57/2017). MA was supported in part by grants from the National Institute of Environmental Health Sciences (NIEHS R01ES07331, NIEHS R01ES10563 and NIEHS R01ES020852). Preparation of this review was conducted during the normal course of the author’s scientific activity as supported by their institutions.
Footnotes
Declaration of Interest
The authors report no conflict of interest.
References
- Abu Khweek A, Amer AO. 2018. Factors Mediating Environmental Biofilm Formation by Legionella pneumophila. Front Cell Infect Microbiol [Internet] 8:1–10. Available from: http://journal.frontiersin.org/article/10.3389/fcimb.2018.00038/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altındag A, Ergönül MB, Yigit S, Baykan Ö. 2008. The acute toxicity of lead nitrate on Daphnia magna Straus. African J Biotechnol [Internet] 7:4298–4300. Available from: http://www.academicjournals.org/AJB [Google Scholar]
- Anbalagan C, Lafayette I, Antoniou-Kourounioti M, Gutierrez C, Martin JR, Chowdhuri DK, De Pomerai DI. 2013. Use of transgenic GFP reporter strains of the nematode Caenorhabditis elegans to investigate the patterns of stress responses induced by pesticides and by organic extracts from agricultural soils. Ecotoxicology 22:72–85. [DOI] [PubMed] [Google Scholar]
- Anbalagan C, Lafayette I, Antoniou-Kourounioti M, Haque M, King J, Johnsen B, Baillie D, Gutierrez C, Martin JAR, Pomerai D De. 2012. Transgenic nematodes as biosensors for metal stress in soil pore water samples. Ecotoxicology 21:439–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GL, Boyd WA, Williams PL. 2001. Assessment of sublethal endpoints for toxicity testing with the nematode Caenorhabditis elegans. Environ Toxicol Chem 20:833–838. [PubMed] [Google Scholar]
- Anderson GL, Cole RD, Williams PL. 2004. Assessing behavioral toxicity with Caenorhabditis elegans. Environ Toxicol Chem 23:1235–1240. [DOI] [PubMed] [Google Scholar]
- ASTM International. 2014. Standard guide for conducting laboratory soil toxicity tests with the nematode Caenorhabditis elegans E 2172–01.
- Bailey DC, Todt CE, Burchfield SL, Pressley AS, Denney RD, Snapp IB, Negga R, Traynor WL, Fitsanakis VA. 2018. Chronic exposure to a glyphosate-containing pesticide leads to mitochondrial dysfunction and increased reactive oxygen species production in Caenorhabditis elegans. Environ Toxicol Pharmacol 57:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DC, Todt CE, Orfield SE, Denney RD, Snapp IB, Negga R, Montgomery KM, Bailey AC, Pressley AS, Traynor WL, Fitsanakis VA. 2016. Caenorhabditis elegans chronically exposed to a Mn/Zn ethylene-bis-dithiocarbamate fungicide show mitochondrial Complex I inhibition and increased reactive oxygen species. Neurotoxicology 56:170–179. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Pistollato F, Sachana M, Bopp SK, Munn S, Worth A. 2018. Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods. Toxicol Appl Pharmacol 354:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Santo F, Guerra N, Vianna MS, Torres JPM, Marchioro AC, Niemeyer CJ. 2019. Laboratory and field tests for risk assessment of metsulfuron-methyl- based herbicides for soil fauna. Chemosphere 222:645–655. [DOI] [PubMed] [Google Scholar]
- Benedetto A, Au C, Aschner M. 2009. Manganese-induced dopaminergic neurodegeneration: Insights into mechanisms and genetics shared with parkinson’s disease. Chem Rev 109:4862–4884. [DOI] [PubMed] [Google Scholar]
- Benedetto A, Au C, Avila DS, Milatovic D, Aschner M. 2010. Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3 – Dependent Manner in Caenorhabditis elegans. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard F, Brulle F, Dumez S, Lemiere S, Platel A, Nesslany F. 2015. Ecotoxicology and Environmental Safety Antioxidant responses of Annelids, Brassicaceae and Fabaceae to pollutants: A review. Ecotoxicol Environ Saf 114:273–303. [DOI] [PubMed] [Google Scholar]
- Bicho RC, Gomes SIL, Soares AMVM. 2014. Non-avoidance behaviour in enchytraeids to boric acid is related to the GABAergic mechanism. Environ Sci Pollut Res:1–6. [DOI] [PubMed]
- Black MC, Williams PL. 2001. Preliminary Assessment of Metal Toxicity in the Middle Tisza River (Hungary) Flood Plain. J soils Sediments 1:213–216. [Google Scholar]
- Bone AJ, Matson CW, Colman BP, Yang X, Meyer JN, Di Giulio RT. 2015. Silver nanoparticle toxicity to Atlantic killifish (Fundulus heteroclitus) and Caenorhabditis elegans: A comparison of mesocosm, microcosm, and conventional laboratory studies. Environ Toxicol Chem 34:275–282. [DOI] [PubMed] [Google Scholar]
- Bongers T, Ferris H. 1999. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol Evol 14:224–228. [DOI] [PubMed] [Google Scholar]
- Bongers T, Ilieva-Makulec K, Ekschmitt K. 2001. Acute sensitivity of nematode taxa to CuSO4and relationships with feeding-type and life-history classification. Environ Toxicol Chem 20:1511–1516. [DOI] [PubMed] [Google Scholar]
- Boyd W, Smith MV, Kissling GE, Freedman JH. 2010. Medium- and high-throughput screening of neurotoxicants using C. elegans. Neurotoxicol Teratol 32:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WA, Anderson GL, Dusenbery DB, Williams PL. 2000. Computer tracking method for assessing behavioral changes in the nematode Caenorhabditis elegans. Environ Toxicol Risk Assess Recent Achiev Environ Fate Transp Ninth Vol. 1381:225–238. [Google Scholar]
- Boyd WA, Williams PL. 2003a. Comparison of the sensitivity of three nematode species to copper and their utility in aquatic and soil toxicity tests. Environ Toxicol Chem 22:2768–2774. [DOI] [PubMed] [Google Scholar]
- Boyd WA, Williams PL. 2003b. Availability of metals to the nematode Caenorhabditis elegans: Toxicity based on total concentrations in soil and extracted fractions. Environ Toxicol Chem 22:1100–1106. [PubMed] [Google Scholar]
- Brenner S 1974. The genetics of Caenorhabditis elegans. Genetics 77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brix KV, Schlekat CE, Garman ER 2017. The mechanisms of nickel toxicity in aquatic environments: an adverse outcome pathway analysis. Environ Toxicol Chem 36:1128–1137. [DOI] [PubMed] [Google Scholar]
- Canada. 2018a. The Regulation of Pesticides in Canada [Internet] [cited 2018 Sep 18]. Available from: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/pesticides-pest-management/fact-sheets-other-resources/regulation-pesticides.html
- Canada. 2018b. Regulatory Proposal PRO2017–01, Cumulative Risk Assessment Framework [Internet] [cited 2018 Sep 18]. Available from: https://www.canada.ca/en/health-canada/services/consumer-product-safety/pesticides-pest-management/public/consultations/regulatory-proposals/2017/cumulative-risk-assessment-framework/document.html
- Castro BB, Silva C, Macário IPE, Oliveira B, Gonçalves F, Pereira JL. 2018. Feeding inhibition in Corbicula fluminea (O.F. Muller, 1774) as an effect criterion to pollutant exposure: Perspectives for ecotoxicity screening and refinement of chemical control. Aquat Toxicol 196:25–34. [DOI] [PubMed] [Google Scholar]
- Caswell-Chen EP, Chen J, Lewis EE, Douhan GW, Nadler SA, Carey JR. 2005. Revising the Standard Wisdom of C. elegans Natural History: Ecology of Longevity. Sci Aging Knowl Environ; 40:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Martinez-Finley EJ, Bornhorst J, Chakraborty S, Aschner M. 2013. Metal-induced neurodegeneration in C. elegans. Front Aging Neurosci 5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioci LK, Qiu L, Freedman JH. 2000. Transgenic strains of the nematode Caenorhabditis elegans as biomonitors of metal contamination. Environ Toxicol Chem 19:2122–2129. [Google Scholar]
- Cole RD, Anderson GL, Williams PL. 2004. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol Appl Pharmacol 194:248–256. [DOI] [PubMed] [Google Scholar]
- Collin B, Tsyusko OV, Starnes DL, Unrine JM 2016. Effect of natural organic matter on dissolution and toxicity of sulfidized silver nanoparticles to Caenorhabditis elegans. Environ Sci Nano 3:728–736. [Google Scholar]
- Corsi AK, Wightman B, Chalfie M. 2015. A Transparent window into biology: A primer on Caenorhabditis elegans. In: Wormbook C elegans Res Community [Internet]: Wormbook; p. 1–31. Available from: http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custodia N, Won SJ, Novillo A, Wieland M, Li C, Callard IP. 2001. Caenorhabditis elegans as an Environmental Monitor Using DNA Microarray Analysis. Ann N Y Acad Sci 948:32–42. [DOI] [PubMed] [Google Scholar]
- Daam MA, Leitão S, Cerejeira MJ, Paulo Sousa J. 2011. Comparing the sensitivity of soil invertebrates to pesticides with that of Eisenia fetida. Chemosphere 85:1040–1047. [DOI] [PubMed] [Google Scholar]
- Destefani A, Costa D, Zanardo T, Taufner G. 2017. Discovering Capacity in Novel Targets: C. Elegans as a Model Life Form in Toxicological Research. J Clin Exp Dermatol Res 07:0–21. [Google Scholar]
- Dhawan R, Dusenbery DB, Williams PL, Hawan RITUD, Usenbery DABD, Illiams PHLW. 2000. A comparison of metal-induced lethality and behavioral response in the nematode Caenorhabditis elegans. Environ Toxicol Chem 19:3061–3067. [Google Scholar]
- EC. 2009. Regulation (EC) No 1107/2009 of the European Parliament and of the Coucil. Off J Eur Union 309:1–50. [Google Scholar]
- EC. 2018. Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH):1–512.
- EFSA. 2009. Scientific Opinion of the Scientific Committee “Existing approaches incorporating replacement, reduction and refinement of animal testing: applicability in food and feed risk assessment”. EFSA J 1052: 1–77. [Google Scholar]
- EFSA. 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge-of-field surface waters. EFSA J 11:3290. [Google Scholar]
- EFSA. 2017. Scientific Opinion addressing the state of the science on risk assessment of plant protection products for in-soil organisms. EFSA J 15:1–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. 1998. Guidelines for Neurotoxicity Risk Assessment 63:1–78. [Google Scholar]
- EPA. 2018a. Pesticide Registration [Internet] [cited 2018 Sep 18]. Available from: https://www.epa.gov/pesticide-registration/about-pesticide-registration
- EPA. 2018b. Exposure Assessment Tools by Tiers and Types - Screening-Level and Refined [Internet] [cited 2018 Sep 18]. Available from: https://www.epa.gov/expobox/exposure-assessment-tools-tiers-and-types-screening-level-and-refined
- EPA. 2018c. TSCA Chemical Substance Inventory [Internet] [cited 2018 Dec 4]. Available from: https://www.epa.gov/tsca-inventory
- EPA. 2018d. Resource Conservation and Recovery Act (RCRA) Laws and Regulations [Internet] [cited 2018 Dec 4]. Available from: https://www.epa.gov/rcra
- EPA. 2019. Summary of the Toxic Substances Control Act. 2016 Chemical Law Updates the TSCA [Internet] Available from: https://www.epa.gov/laws-regulations/summary-toxic-substances-control-act.
- European Commission. 2006. EC regulation No 1881/2006 :5–24.
- Fagundez DDA, Câmara DF, Salgueiro WG, Noremberg S, Luiz Puntel R, Piccoli JE, Garcia SC, da Rocha JBT, Aschner M, Ávila DS. 2015. Behavioral and dopaminergic damage induced by acute iron toxicity in Caenorhabditis elegans. Toxicol Res 4:878–884. [Google Scholar]
- Farina M, Silva Avila D, Teixera da Rocha JB, Aschner M. 2014. Metals, Oxidative Stress and Neurodegeneration: A focus on Iron, Manganese and Mercury. Neurochem Int 62:575–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira ALG, Loureiro S, Soares AMVM. 2008. Toxicity prediction of binary combinations of cadmium, carbendazim and low dissolved oxygen on Daphnia magna. Aquat Toxicol 89:28–39. [DOI] [PubMed] [Google Scholar]
- Fire AZ. 2007. Gene Silencing by Double-Stranded RNA (Nobel Lecture) England: Int. Ed. [DOI] [PubMed] [Google Scholar]
- Frézal L, Félix M-A. 2015. The Natural History of Model Organisms: C. elegans outside the Petri dish. eLife 4:e05849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche E 2017. Meeting report OECD / EFSA Workshop on Developmental Neurotoxicity (DNT): The Use of Non-Animal Test Methods for Regulatory Purposes 34:311–315. [DOI] [PubMed] [Google Scholar]
- Fritsche E, Alm H, Baumann J, Geerts L, Håkansson H, Witters H. 2015. External scientific report. Literature review on in vitro and alternative Developmental Neurotoxicity. EFSA Support Publ:1–186.
- Fukuto TR. 1990. Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect 87:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer A, Akre R, Owojori OJ, Siciliano SD. 2019. Chemosphere Protecting vulnerable individuals in a population: is the avoidance response of juvenile soil invertebrates more sensitive than the adults response? Chemosphere 220:658–667. [DOI] [PubMed] [Google Scholar]
- Graves AL, Boyd WA, Williams PL. 2005. Using transgenic Caenorhabditis elegans in soil toxicity testing. Arch Environ Contam Toxicol 48:490–494. [DOI] [PubMed] [Google Scholar]
- Habchi J, Chia S, Limbocker R, Mannini B, Ahn M, Perni M, Hansson O. 2016. Systematic development of small molecules to inhibit specific microscopic steps of Aβ42 aggregation in Alzheimer ‘s disease. PNAS:200–208. [DOI] [PMC free article] [PubMed]
- Haegerbaeumer A, Höss S, Ristau K, Claus E, Möhlenkamp C, Heininger P, Traunspurger W. 2016. A comparative approach using ecotoxicological methods from single-species bioassays to model ecosystems. Environ Toxicol Chem 35:2987–2997. [DOI] [PubMed] [Google Scholar]
- Hagerbaumer A, Sebastian H, Heininger P, Traunspurger W. 2015. Experimental Studies with Nematodes in Ecotoxicology: An Overview. J Nematol 47:11–27. [PMC free article] [PubMed] [Google Scholar]
- Harada H, Kurauchi M, Hayashi R, Eki T. 2007. Shortened lifespan of nematode Caenorhabditis elegans after prolonged exposure to heavy metals and detergents. Ecotoxicol Environ Saf 66:378–383. [DOI] [PubMed] [Google Scholar]
- Harrington AJ, Hamamichi S, Caldwell GA, Caldwell KA. 2010. C. elegans as a model organism to investigate molecular pathways involved with Parkinson’s disease. Dev Dyn 239:1282–1295. [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Horvitz HR. 1994. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 76:665–676. [DOI] [PubMed] [Google Scholar]
- Horvitz HR. 1999. Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Cancer Res 59:1701S–1706S. [PubMed] [Google Scholar]
- Hoshi H, Kamata Y, Uemura T. 2003. Effects of 17beta-estradiol, bisphenol A and tributyltin chloride on germ cells of Caenorhabditis elegans. J Vet Med Sci 65:881–885. [DOI] [PubMed] [Google Scholar]
- Höss S, Arndt M, Baumgarte S, Tebbe CC, Nguyen HT, Jehle JA. 2008. Effects of transgenic corn and Cry1Ab protein on the nematode, Caenorhabditis elegans. Ecotoxicol Environ Saf 70:334–340. [DOI] [PubMed] [Google Scholar]
- Höss S, Haitzer M, Traunspurger W, Steinberg CEW. 1999. Growth and Fertility of Caenorhabditis elegans (Nematoda) in Unpolluted Freshwater Sediments: Response To Particle Size Distribution and Organic Content. Environ Toxicol Chem 18:2921–2925. [Google Scholar]
- Höss S, Henschel T, Haitzer M, Traunspurger W, Steinberg CE. 2001. Toxicity of cadmium to Caenorhabditis elegans (Nematoda) in whole sediment and pore water - the ambiguous role of organic matter. Environ Toxicol Chem 20:2794–2801. [PubMed] [Google Scholar]
- Höss S, Jänsch S, Moser T, Junker T, Römbke J. 2009. Assessing the toxicity of contaminated soils using the nematode Caenorhabditis elegans as test organism. Ecotoxicol Environ Saf 72:1811–1818. [DOI] [PubMed] [Google Scholar]
- Höss S, Jüttner I, Traunspurger W. 2002. Enhanced growth and reproduction of Caenorhabditis elegans (Nematoda) in the presence of 4-Nonylphenol. Environ Pollut 120:169–172. [DOI] [PubMed] [Google Scholar]
- Höss S, Nguyen HT, Menzel R, Pagel-Wieder S, Miethling-Graf R, Tebbe CC, Jehle JA, Traunspurger W. 2011. Assessing the risk posed to free-living soil nematodes by a genetically modified maize expressing the insecticidal Cry3Bb1 protein. Sci Total Environ 409:2674–2684. [DOI] [PubMed] [Google Scholar]
- Huang CW, Li SW, Hsiu-Chuan Liao V. 2017. Chronic ZnO-NPs exposure at environmentally relevant concentrations results in metabolic and locomotive toxicities in Caenorhabditis elegans. Environ Pollut 220:1456–1464. [DOI] [PubMed] [Google Scholar]
- Hunt PR. 2016. The C. elegans model in toxicity testing. J Appl Toxicol:50–59. [DOI] [PMC free article] [PubMed]
- Ibrahim AM, Sayed DA. 2019. Toxicological impact of oxyfluorfen 24% herbicide on the reproductive system, antioxidant enzymes, and endocrine disruption of Biomphalaria alexandrina (Ehrenberg, 1831) snails. Environ Sci Pollut Res 26:7960–7968. [DOI] [PubMed] [Google Scholar]
- ISO. ISO 11267. Soil quality - Inhibition of reproduction of Collembola (Folsomia candida) by soil pollutants. 1999.
- ISO. 2008. ISO 17512–1 Soil quality – avoidance test for determining the quality of soils and effects of chemicals on behaviour-Part 1: Test with earthworms (Eisenia fetida and Eisenia andrei). In: Geneva, Switzerland: International Organization for Standardization. [Google Scholar]
- ISO. ISO 10872 Water quality — determination of the toxic effect of sediment and soil samples on growth, fertility and reproduction of Caenorhabditis elegans (Nematoda). 2010.
- Jiang Y, Chen J, Wu Y, Wang Q, Li H. 2016. Sublethal toxicity endpoints of heavy metals to the nematode Caenorhabditis elegans. PLoS One 11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker MJ, Piskiewicz AM, Ivorra I Castellà N, Kammenga JE. 2004. Toxicity of binary mixtures of cadmium-copper and carbendazim-copper to the nematode Caenorhabditis elegans. Environ Toxicol Chem 23:1529–1537. [DOI] [PubMed] [Google Scholar]
- Jonker MJ, Sweijen RAJC, Kammenga JE. 2004. Toxicity of simple mixtures to the nematode Caenorhabditis elegans in relation to soil sorption. Environ Toxicol Chem 23:480–488. [DOI] [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. 2006. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov:1–12. [DOI] [PubMed]
- Kim HJ, Koedrith P, Seo YR. 2015. Ecotoxicogenomic Approaches for Understanding Molecular Mechanisms of Environmental Chemical Toxicity Using Aquatic Invertebrate, Daphnia Model Organism. Int J Mol Sci 16:12261–12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiontke K 2006. Ecology of Caenorhabditis species. WormBook [Internet]:1–14. Available from: http://www.wormbook.org/chapters/www_ecolCaenorhabditis/ecolCaenorhabditis.html [DOI] [PMC free article] [PubMed]
- Kurauchi M, Morise H, Eki T. 2009. Using the Nematode Caenorhabditis elegans daf-16 Mutant to Assess the Lifespan Toxicity of Prolonged Exposure to Ecotoxic Agents. J Heal Sci 55:796–804. [Google Scholar]
- Lavelle P, Decaëns T, Aubert M, Barot S, Blouin M, Bureau F, Margerie P, Mora P, Rossi JP. 2006. Soil invertebrates and ecosystem services. Eur J Soil Biol 42:S3–S15. [Google Scholar]
- Leelaja BC, Rajini PS. 2013. Biochemical and physiological responses in Caenorhabditis elegans exposed to sublethal concentrations of the organophosphorus insecticide, monocrotophos. Ecotoxicol Environ Saf 94:8–13. [DOI] [PubMed] [Google Scholar]
- Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, Shi H, Raley-Susman KM, He D. 2018. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ 619–620:1–8. [DOI] [PubMed] [Google Scholar]
- Leung MCK, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. 2008. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol Sci 106:5–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JA, Gehman EA, Baer CE, Jackson DA. 2013. Alterations in gene expression in Caenorhabditis elegans associated with organophosphate pesticide intoxication and recovery. BMC Genomics 14:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wei L, Cao J, Qiu L, Jiang X, Li P, Song Q. 2016. Oxidative stress, DNA damage and antioxidant enzyme activities in the pacific white shrimp (Litopenaeus vannamei) when exposed to hypoxia and reoxygenation. Chemosphere 144:234–240. [DOI] [PubMed] [Google Scholar]
- Loureiro S, Svendsen C, Ferreira ALG, Pinheiro C, Ribeiro F, Soares AMVM. 2010. Toxicity of three binary mixtures to Daphnia magna: Comparing chemical modes of action and deviations from conceptual models. Environ Toxicol Chem 29:1716–1726. [DOI] [PubMed] [Google Scholar]
- Lublin AL, Link CD. 2014. Alzheimer’s Disease Drug Discovery: In-vivo screening using C. elegans as a model for β-amyloid peptide-induced toxicity. Drug Discov Today Technol 10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Glenn TC, Jagoe CH, Jones KL, Williams PL. 2009. A transgenic strain of the nematode Caenorhabditis elegans as a biomonitor for heavy metal contamination. Environ Toxicol Chem 28:1311–1318. [DOI] [PubMed] [Google Scholar]
- Madden JC, Hewitt M, Przybylak K, Vandebriel RJ, Piersma AH, Cronin MTD. 2013. Strategies for the optimisation of in vivo experiments in accordance with the 3Rs philosophy. Regul Toxicol Pharmacol 63:140–154. [DOI] [PubMed] [Google Scholar]
- Majdi N, Traunspurger W. 2015. Free-Living Nematodes in the Freshwater Food Web: A Review. J Nematol 47:28–44. [PMC free article] [PubMed] [Google Scholar]
- Mallmann GC, Sousa JP, Sundh I, Pieper S, Arena M, da Cruz SP, Klauberg-Filho O. 2018. Placing arbuscular mycorrhizal fungi on the risk assessment test battery of plant protection products (PPPs). Ecotoxicology:1–10. [DOI] [PubMed]
- Mcmahon JW. 1965. Some physical factors influencing the feeding behavior of Daphnia magna Straus. 43:603–611. [Google Scholar]
- Mcvey KA, Mink JA, Snapp IB, Timberlake WS, Todt CE, Negga R, Fitsanakis VA. 2012. Caenorhabditis elegans: an emerging model system for pesticide neurotoxicity. J Environ Anal Toxicol S4:003. [Google Scholar]
- Mello CC. 2007. Return to the RNAi world: rethinking gene expression and evolution. Cell Death Differ:2013–2020. [DOI] [PubMed]
- Menzel R, Rödel M, Kulas J, Steinberg CEW. 2005. CYP35: Xenobiotically induced gene expression in the nematode Caenorhabditis elegans. Arch Biochem Biophys 438:93–102. [DOI] [PubMed] [Google Scholar]
- Menzel R, Swain SC, Hoess S, Claus E, Menzel S, Steinberg CEW, Reifferscheid G, Stürzenbaum SR. 2009. Gene expression profiling to characterize sediment toxicity - A pilot study using Caenorhabditis elegans whole genome microarrays. BMC Genomics 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Williams PL. 2014. Toxicity testing of neurotoxic pesticides in Caenorhabditis elegans. J Toxicol Environ Heal - Part B Crit Rev 17:284–306. [DOI] [PubMed] [Google Scholar]
- Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K. 2015. Neonicotinoid contamination of surface waters and relative risks to aquatic invertebrates: a review. Environ Int 74:291–303. [DOI] [PubMed] [Google Scholar]
- Moyson S, Vissenberg K, Fransen E, Blust R, Husson SJ. 2018. Mixture Effects of Copper, Cadmium, and Zinc on Mortality and Behavior of C. elegans. Environ Toxicol Chem 37:145–159. [DOI] [PubMed] [Google Scholar]
- Mutwakil MHAZ, Reader JP, Holdich DM, Smithurst PR, Candido EPM, Jones D, Stringham EG. 1997. Use of Stress-Inducible Transgenic Nematodes as Biomarkers of Heavy Metal Pollution in Water Samples from an English River System. Arch Environ Contam Toxicol 153:146–153. [DOI] [PubMed] [Google Scholar]
- Nam SH, An YJ. 2010. Assessing the ecotoxicity of vinyl chloride using green alga P. subcapitata, nematode C. elegans, and the SOS chromotest in a closed system without headspace. Sci Total Environ 408:3148–3152. [DOI] [PubMed] [Google Scholar]
- Negga R, Rudd DA, Davis NS, Justice AN, Hatfield HE, Valente AL, Fields AS, Fitsanakis VA. 2011. Exposure to Mn/Zn Ethylene-bis-Dithiocarbamate and Glyphosate Pesticides Leads to Neurodegeneration in Caenorhabditis elegans. Neurotoxicology 32:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negga R, Stuart JA, Machen ML, Salva J, Lizek AJ, Richardson SJ, Osborne AS, Mirallas O, Mcvey KA, Fitsanakis VA. 2012. Exposure to Glyphosate- and/or Mn/Zn-Ethylene-bis- Dithiocarbamate-Containing Pesticides Leads to Degeneration of γ-Aminobutyric Acid and Dopamine Neurons in Caenorhabditis elegans. Neurotox Res 21:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigon V, Dougherty E C 1949. Reproductive patterns and attempts at reciprocal crossing of Rhabditis elegans Maupas, 1900, and Rhabditis briggsae Dougherty and Nigon, 1949 (nematoda Rhabditidae). :485–503. [DOI] [PubMed]
- Novillo A, Won S-J, Li C, Callard IP. 2005. Changes in Nuclear Receptor and Vitellogenin Gene Expression in Response to Steroids and Heavy Metal in Caenorhabditis elegans. IntegrCompBiol 45:61–71. [DOI] [PubMed] [Google Scholar]
- OECD. 1992. OECD guideline for testing of chemicals. Fish, acute toxicity test. Test N203:1–10.
- OECD. 2000. OECD guideline for testing of chemicals. Test No. 216: Soil Microorganisms: Nitrogen Transformation Test:1–10.
- OECD. 2004a. OECD Guidelines for the Testing of Chemicals, Daphnia sp., Acute Immobilisation Test. Test N202 :1–12.
- OECD. 2004b. OECD guidelines for the testing of Chemicals. Predatory mite (Hypoaspis (Geolaelaps) aculeifer) reproduction test in soil. Test N226 :1–21.
- OECD. 2006. OECD Guidelines for the testing of chemicals. Lemna sp. Growth Inhibition Test. Test N221. :1–22.
- OECD. 2007a. OECD Guidelines for the testing of chemicals. Sediment-Water Lumbriculus Toxicity Test Using Spiked Sediment. Test N225 :1–31.
- OECD. 2007b. OECD Guideline for the testing of chemicals. Developmental Neurotoxicity Study. Test N426 :1–26.
- OECD. 2008. OECD guidelines for the testing of chemicals: Daphnia magna Reproduction Test. Test N211 :1–23.
- OECD. 2009. OECD Guidelines for testing chemical. Collembolan Reproduction Test in Soil. Test N 232:1–19.
- OECD. 2011a. OECD Guidelines for the testing of Chemicals. Freshwater Alga and Cyanobacteria, Growth Inhibition Test. Test N201 :1–25.
- OECD. 2011b. OECD guideline for the testing of chemicals. Chironomus sp., Acute Immobilisation Test. Test N235.:1–17.
- OECD. 2015. OECD Guideline for the Testing of Chemicals. Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei).:1–18.
- Pelaez V, da Silva LR, Araújo EB. 2013. Regulation of pesticides: A comparative analysis. Sci Public Policy 40:644–656. [Google Scholar]
- Pelosi C, Barot S, Capowiez Y, Hedde M, Vandenbulcke F. 2014. Pesticides and earthworms. A review. Agron Sustain Dev 34:199–228. [Google Scholar]
- Peredney CL, Williams PL. 2000. Utility of Caenorhabditis elegans for assessing heavy metal contamination in artificial soil. Arch Environ Contam Toxicol 39:113–118. [DOI] [PubMed] [Google Scholar]
- Pereira JL, Antunes SC, Ferreira AC, Goncalves F, Pereira R. 2010. Avoidance behavior of earthworms under exposure to pesticides: is it always chemosensorial? J Environ Sci Health B 45:229–32. [DOI] [PubMed] [Google Scholar]
- Petrie B, Barden R, Kasprzyk-Hordern B. 2014. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res 72:3–27. [DOI] [PubMed] [Google Scholar]
- Qi S, Wang D, Zhu L, Teng M, Wang C, Xue X, Wu L. 2018. Neonicotinoid insecticides imidacloprid, guadipyr, and cycloxaprid induce acute oxidative stress in Daphnia magna. Ecotoxicol Environ Saf 148:352–358. [DOI] [PubMed] [Google Scholar]
- Rasch J, Krüger S, Fontvieille D, Ünal CM, Michel R, Labrosse A, Steinert M. 2016. Legionella-protozoa-nematode interactions in aquatic biofilms and influence of Mip on Caenorhabditis elegans colonization. Int J Med Microbiol 306:443–451. [DOI] [PubMed] [Google Scholar]
- Reichert K, Menzel R. 2005. Expression profiling of five different xenobiotics using a Caenorhabditis elegans whole genome microarray. Chemosphere 61:229–237. [DOI] [PubMed] [Google Scholar]
- Reinecke SA, Reinecke AJ. 2007. The impact of organophosphate pesticides in orchards on earthworms in the Western Cape, South Africa. Ecotoxicol Environ Saf 66:244–251. [DOI] [PubMed] [Google Scholar]
- Roh J-Y, Sim SJ, Yi J, Park K, Chung KH, Ryu D-YY, Choi J. 2009. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Env Sci Technol 43:3933–3940. [DOI] [PubMed] [Google Scholar]
- Roh JY, Choi J. 2008. Ecotoxicological evaluation of chlorpyrifos exposure on the nematode Caenorhabditis elegans. Ecotoxicol Environ Saf 71:483–489. [DOI] [PubMed] [Google Scholar]
- Roh JY, Jung IH, Lee JY, Choi J. 2007. Toxic effects of di(2-ethylhexyl)phthalate on mortality, growth, reproduction and stress-related gene expression in the soil nematode Caenorhabditis elegans. Toxicology 237:126–133. [DOI] [PubMed] [Google Scholar]
- Roh JY, Lee J, Choi J. 2006. Assessment of stress-related gene expression in the heavy metal-exposed nematode Caenorhabditis elegans: A potential biomarker for metal-induced toxicity monitoring and environmental risk assessment. Environ Toxicol Chem 25:2946–2956. [DOI] [PubMed] [Google Scholar]
- Roh JY, Park YK, Park K, Choi J. 2010. Ecotoxicological investigation of CeO2and TiO2nanoparticles on the soil nematode Caenorhabditis elegans using gene expression, growth, fertility, and survival as endpoints. Environ Toxicol Pharmacol 29:167–172. [DOI] [PubMed] [Google Scholar]
- Schulenburg H, Félix MA. 2017. The natural biotic environment of Caenorhabditis elegans. Genetics 206:55–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões T, Novais SC, Natal-da-luz T, Devreese B, Sousa P, Straalen NM Van, Lemos MFL, Boer T De, Roelofs D. 2019. Using time-lapse omics correlations to integrate toxicological pathways of a formulated fungicide in a soil invertebrate. Environ Pollut 246:845–854 [DOI] [PubMed] [Google Scholar]
- Sneddon LU, Halsey LG, Bury NR. 2017. Considering aspects of the 3Rs principles within experimental animal biology. J Exp Biol:3007–3016. [DOI] [PubMed]
- Sochová I, Hofman J, Holoubek. 2007. Effects of seven organic pollutants on soil nematode Caenorhabditis elegans. 33:798–804. [DOI] [PubMed] [Google Scholar]
- Sochová I, Hofman J, Holoubek I. 2006. Using nematodes in soil ecotoxicology. Environ Int 32:374–383. [DOI] [PubMed] [Google Scholar]
- Sorvari J, Sillanpää M. 1996. Influence of metal complex formation on heavy metal and free EDTA and DTPA acute toxicity determined by Daphnia magna. Chemosphere 33:1119–1127. [Google Scholar]
- Spurgeon DJ, Hopkin SP, Jones DT. 1994. Effects of cadmium, copper, lead and zinc on growth, reproduction and survival of the earthworm Eisenia fetida (Savigny): Assessing the environmental impact of point-source metal contamination in terrestrial ecosystems. Environ Pollut 84:123–130. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Leitch DB, Savchenko V, Wu J, Fitsanakis VA, Anderson DJ, Stankowski JN, Aschner M, McLaughlin B. 2009. Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. J Neurochem 110:378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streets DG, Qiang Z, Ye W. 2009. Projections of Global Mercury Emissions in 2050. Environ Sci Technol 43:2983–2988. [DOI] [PubMed] [Google Scholar]
- Tejeda-Benitez L, Flegal R, Odigie K, Olivero-Verbel J. 2016. Pollution by metals and toxicity assessment using Caenorhabditis elegans in sediments from the Magdalena River, Colombia. Environ Pollut 212:238–250. [DOI] [PubMed] [Google Scholar]
- Todt CE, Bailey DC, Pressley AS, Orfield SE, Denney RD, Snapp IB, Negga R, Bailey AC, Montgomery KM, Traynor WL, Fitsanakis VA. 2016. Acute exposure to a Mn/Zn ethylene-bis-dithiocarbamate fungicide leads to mitochondrial dysfunction and increased reactive oxygen species production in Caenorhabditis elegans. Neurotoxicology 57:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga N, Tomoeda M, Kohra S, Takao Y, Nagae M, Ueda K, Ishibashi H, Kai T, Arizono K. 2002. A convenient sublethal assay of alkylphenol and organotin compounds using the nematode Caenorhabditis elegans. J Heal Sci 48:555–559. [Google Scholar]
- Tominaga N, Ura K, Kawakami M, Kawaguchi T, Kohra S, Mitsui Y, Iguchi T, Arizono K. 2003. Caenorhabditis elegans responses to specific steroid hormones. J Heal Sci 49:28–33. [Google Scholar]
- Traunspurger W, Haitzer M, Höss S, Beier S, Ahlf W, Steinberg C. 1997. Ecotoxicological assessment of aquatic sediments with Caenorhabditis elegans (nematoda) - a method for testing liquid medium and whole-sediment samples. Environ Toxicol Chem 16:245–250. [Google Scholar]
- U.S. Environmental Protection Agency ECOTOX database. U.S. Environmental Protection Agency ECOTOX database [Internet] 2018 Available from: https://cfpub.epa.gov/ecotox/ecotox_home.cfm.
- De Vaufleury A, Cœurdassier M, Pandard P, Scheifler R, Lovy C, Crini N, Badot PM. 2006. How terrestrial snails can be used in risk assessment of soils. Environ Toxicol Chem 25:797–806. [DOI] [PubMed] [Google Scholar]
- Vidal T, Santos JI, Queirós L, Ré A, Abrantes N, Gonçalves FJM, Pereira JL. 2019. Environmental benchmarks based on ecotoxicological assessment with planktonic species might not adequately protect benthic assemblages in lotic systems. Sci Total Environ 668:1289–1297. [DOI] [PubMed] [Google Scholar]
- Wah Chu K, Chow KL. 2002. Synergistic toxicity of multiple heavy metals is revealed by a biological assay using a nematode and its transgenic derivative. Aquat Toxicol 61:53–64. [DOI] [PubMed] [Google Scholar]
- Wang H, Wick RL, Xing B. 2009. Toxicity of nanoparticulate and bulk ZnO, Al2O3and TiO2to the nematode Caenorhabditis elegans. Environ Pollut 157:1171–1177. [DOI] [PubMed] [Google Scholar]
- Wang X, Anadón A, Wu Q, Qiao F, Ares I, Yuan Z. 2018. Mechanism of Neonicotinoid Toxicity: Impact on Oxidative Stress and Metabolism. Annu Rev Pharmacol Toxicol 58:471–507. [DOI] [PubMed] [Google Scholar]
- Windsor FM, Ormerod SJ, Tyler CR. 2018. Endocrine disruption in aquatic systems: up-scaling research to address ecological consequences. Biol Rev 93:626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YF, Cheng YH, Liao CM. 2017. Nematode-based biomarkers as critical risk indicators on assessing the impact of silver nanoparticles on soil ecosystems. Ecol Indic 75:340–351. [Google Scholar]
- Yuan JY, Shaham S, Ledoux S, Ellis HM, Horvitz HR. 1993. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1B-converting enzyme. Cell 75:641–652. [DOI] [PubMed] [Google Scholar]
- Zeng R, Yu X, Tan X, Ye S, Ding Z. 2017. Deltamethrin affects the expression of voltage-gated calcium channel α1 subunits and the locomotion, egg-laying, foraging behavior of Caenorhabditis elegans. Pestic Biochem Physiol 138:84–90. [DOI] [PubMed] [Google Scholar]
- Zhou D, Yang J, Li H, Cui C, Yu Y, Liu Y, Lin K. 2016. The chronic toxicity of bisphenol A to Caenorhabditis elegans after long-term exposure at environmentally relevant concentrations. Chemosphere 154:546–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


